Emulsions are some of the most common vehicles used in skin care due to their strong affinity to skin and because they can provide a variety of formulation benefits. They have the ability to incorporate hydrophilic and lipophilic actives in the same product, generate vehicles with different textures and viscosities, and facilitate the permeation of active ingredients through the skin.1, 2
However, emulsions are thermodynamically unstable systems. Therefore, in an exploratory study of three test emulsions, the present article examines two physical attributes: zeta potential and particle size distribution, and their ability to screen and potentially predict the instabilities of the emulsions. These methods are suggested to supplement regular accelerated stability testing during product development.
Emulsion Dynamics
Emulsion systems consist of at least two immiscible liquid phases, one of which is dispersed as globules in the other.3 These phases are basically composed of three components: water, oil and emulsifier, whose physicochemical properties affect the behavior of the system as a whole. As most formulators know, emulsion systems can be classified as oil-in-water (o/w), when the dispersed phase is oil and the external phase is water; or water-in-oil (w/o), in the opposite case. More complex, multiple-type systems, such as o/w/o and w/o/w emulsions, are also used in the cosmetics industry.4-6
Emulsifiers are organic compounds that are capable of influencing the stability of colloidal systems by modifying the interface between two immiscible phases.7 All emulsifiers are amphiphilic, with a polar portion that is hydrophilic and soluble in water, and a nonpolar portion that is lipophilic and insoluble in water. Emulsifiers are classified on the basis of their hydrophilic or solubilizing groups into four categories: anionic, nonionic, cationic and amphoteric.8
To produce a stable emulsion, an emulsifying agent must be added to the oil and water. An emulsifier will either act as a barrier to alter the droplet coalescence rate, or create an interfacial film that can produce repulsive electrical forces between approaching droplets. With an ionic emulsifier, a monolayer of surfactant is absorbed, and a double layer is built up around the droplets. The double layer consists of the charged portion of the emulsifier at the water interface and, in the case of an anionic emulsifier, the cations surrounding it (see Figure 1).9, 10 At low cation concentrations, the thickness of electrical double layer will increase, activating long-range repulsive forces and causing the droplets to repel one another as they draw together. This potential produced by the double layer creates a repulsive effect between the oil droplets and thus hinders coalescence. This repulsive electrical potential at the emulsion interface can be calculated but not measured directly. It is possible, however, to determine a related quantity–zeta potential.
Zeta potential can be defined as the difference in potential between the surface of the tightly bound layer of ions on the particle surface and the region of the solution. It is, in fact, well-known that when the zeta potential is relatively high, i.e. ≥ 25 mV as an absolute value, the repulsive forces exceed the attractive London forces. Then, particles are dispersed and the system is deflocculated. On the other hand, when the zeta potential is less than 25 mV as an absolute value, attractive forces exceed the repulsive forces and the particles are drawn together and tend to flocculate.9
Predicting Stability
Emulsion stability refers to the ability of an emulsion to resist changes in its properties over time. Physical instability causes changes in the spatial distribution and structural organization of molecules. These changes are characterized, for example, by creaming, flocculation, coalescence, partial coalescence, phase inversion and Ostwald ripening of the emulsion.11-13 Emulsion instability acceleration techniques have been employed to predict the long-term stability of products, and these include: subjecting the formulation to temperature changes as well as temperature cycling (freeze/thaw);14 application of high centrifugal forces to detect separation of the emulsion;15 application of rheological shear stress;16 subjecting the emulsion to vibration; and successive investigation by microscopy.17 Analyses of particle size and zeta potential,18 described next, are other predictive techniques.
Particle Size Analysis
Particle sizes in an emulsion can provide valuable information about its structure. In fact, particle size directly affects the physical stability of emulsions—the smaller the dispersed particles, the stabler the system. Conversely, the faster that particles increase in size, the more unstable the system becomes.19, 20 Stabilizing an emulsion also relates to particle size. To optimize stability, the particles must be small enough to allow a film to form around the droplets in the dispersed phase. Reduced particle size allows for more particles to be distributed on the interface, resulting in a more stable emulsion. However, excessively small particles can be easily removed from the interface and also lead to instability in the formulation.16
Particle size analysis can be conducted using different techniques such as laser diffraction, electrical zone sensing, photon correlation spectroscopy and ultrasonic spectroscopy, although these techniques have some drawbacks. Laser diffraction, electrical zone sensing and photon correlation spectroscopy require diluting the samples, and ultrasonic spectroscopy needs at least 100 mL of sample, which is relatively high.21
Once physical properties are measured, a calculation is used to generate a representative particle size distribution. Some calculation techniques report only a central point and spread of the distribution. Others provide greater detail across the upper and lower particle sizes detected. The particle size distribution can be calculated based on several models, most often as a number or volume/mass distribution. The number of particles corresponds to the actual amount of existing particles, whereas their volume corresponds to the size or space occupied by the particles. For example, 10 particles may occupy volume X, whereas three larger particles may also occupy the same volume X.22
Before making size measurements, it is important to consider the upper and lower detection limits of the instrument being used. Determining the size of particles with irregular surfaces involves the use of the sphere-equivalent principle. This requires establishing a relationship between the property of the particle, i.e., surface area, volume, mass, etc., and the diameter of a sphere of equal size. The particle size is then measured by determining the scattering angle of light striking the particle. This is performed on each particle, and the sum of all the individual patterns form a new pattern. Based on the laws of scattering light, this pattern is converted into a set of numbers for each rated size. The relative amplitude of each number is related to a particle size equivalent of the sphere’s volume. This is the only geometric form that can be represented three-dimensionally by a single number—the diameter.21
Zeta Potential
Another predictive tool is provided by the surface charge of a particle, which is determined with the aid of the zeta potential generated by the electrostatic repulsion from adjacent dispersed particles. This parameter is indicative of the interfacial properties and continuous solution near the interface within an emulsion system. Zeta potential can be used to control the behavior of colloidal suspensions/emulsions and to understand the behavior of their structural components. This includes contributions to the valence of particles, counter-ions and any ions in the shear plane boundary that are interacting with the particles, since the zeta potential is indicative of changes in the surface potential, and the attraction and repulsion forces in colloids.23 In relation, another important emulsifier-related parameter is the free interfacial energy, which can contribute to stability analysis.18
Materials and Methods
As noted, the purpose of this work was to evaluate the stability of cosmetic formulations during their development stage using analyses of zeta potential and particle size distribution. These assessments could be regarded as screening tests, intended to help with the selection of promising formulations prior to accelerated stability, and consequently shortening development time of cosmetic emulsions.
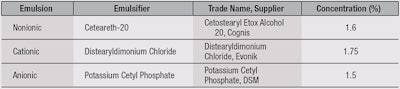
Emulsion preparations: Three test emulsions (see Table 1) were prepared with the same basic components but different ionic properties, and each was adjusted for those characteristics. The formulations included preservatives, different emulsifiers, emollients, polymers, fragrance and water. The aqueous and oil phases were prepared separately by melting at a temperature of 75°C under constant stirring. When two phases reached the same temperature, the oil phase was slowly poured into the aqueous phase.
The products were then removed from the heating plate and continuously stirred until they cooled to room temperature. The pH of each formulation was adjusted with sodium hydroxide and citric acid solutions. The formulations were divided into three groups with approximate pH values of 3.0, 6.0 and 8.0, and stored in appropriate glass bottles. The products were left undisturbed for 24 hr.
Zeta potential determination: The electrophoretic mobilities, or zeta potential, of the manufactured emulsions were measured using a zeta potential analyzera with a 173-degree scattering angle. Measurements were taken at 25°C on samples appropriately diluted in ultrapure waterb. Samples were placed in a specific cell and subjected to a potential of approximately 150 mV. Zeta potentials were calculated using Smoluchowski’s equation based on the mean of the electrophoretic mobility values.18, 24
Particle size and distribution: The size and distribution of particles were determined with laser beam diffractionc in a module for liquids. The liquid used as a carrier for the samples was ultrapure waterb; any air trapped in the water was previously removed by ultrasound treatment for 10 min.
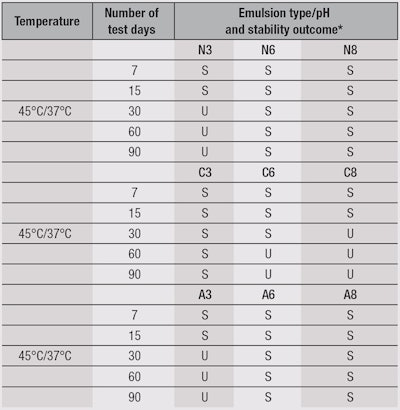
Accelerated stability evaluation: Stability assessments were based on the Cosmetic Products Stability Guide issued by the Agência Nacional de Vigilância Sanitária (ANVISA) in Brazil, the country’s health surveillance agency.15 Analyses were conducted at 1, 7, 15, 30, 60 and 90 days after the preparation of emulsions. Macroscopic changes relative to the baseline standard established for each emulsion were determined visually. The following environmental conditions were used throughout the study: exposure to sunlight, room temperature (23°C), cold storage at 4°C, and heated storage at 37°C and 45°C.15, 20
Statistical analysis: All assays were carried out in triplicate. The measured values of the zeta potential and particle size distribution were compared statistically using an analysis of variance (ANOVA) in combination with the Tukey Test. For all the tests, p values < 0.05 were considered statistically significant.
Results and Discussion
As noted, the emulsions were evaluated by monitoring organoleptic parameters visually, and by analyzing zeta potentials, particle sizes and their distribution. These techniques indicated the test samples became unstable, although with different intensities and response times. As a general rule, fewer numbers of particles indicated stabler systems. Conversely, as dispersed particles increase in size, the system becomes more unstable.18
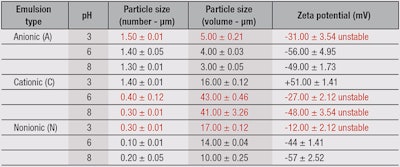
Nonionic emulsions: The results for the nonionic emulsions, listed in Table 2, show that the number of particles remained practically the same while pH values decreased with increasing particle volume. This indicated the particles were clustering and thereby increasing the instability of the system. The zeta potential became more negative as the pH values increased, either because the forces of interaction between dispersed droplets increased and thus decreased their electrostatic repulsion, or because the interfacial film fragility was responsible for steric stabilization.1, 25 Figure 2 also shows that pH values higher than five increased the probability of producing stable emulsions. This condition gives the emulsion a hydrodynamic balance and increases its stability.
Cationic emulsions: In the case of cationic emulsions, the number of particles decreased while pH values increased with increasing particle volume (see Table 2). This is due to the agglomeration of particles, which forms clusters and causes the emulsion to become unstable. Also, cationic emulsifiers have a positive charge when dissociated in water.
These dynamics are corroborated by the results obtained from the zeta potential, which decreased with increasing pH values (see Figure 2). In cationic emulsions, the zeta potential should be positive because lower pH values have an excess of positive charges that can donate protons to substances in the medium. Thus, emulsions C6 and C8 show a negative zeta potential, which suggests instability in the formulations. At higher pH values, cationic emulsifiers tend to become unstable due to an imbalance between the positive charges in the emulsifier and the negative charges in the medium.
Anionic emulsions: In anionic emulsions (see Table 2), there was no difference in the number and volume of particles under different pH values. In anionic emulsions, the zeta potential should be negative because at higher pH values, there is an excess of negative charges that can donate hydroxyl ions to substances in the medium. The emulsion at pH 3 proved to be unstable; it had the lowest zeta potential among all the tested emulsions. At low pH values, anionic emulsifiers tend to become unstable due to the imbalance between negative charges in the emulsifier and positive charges in the medium. As illustrated in Figure 2a, the zeta potential increases with increasing pH values; upwards of pH 7.0, the zeta potential stops rising and reaches a plateau. This instability may engender such phenomena as particle aggregation and disaggregation.
Stability evaluations: As stated, the present investigation evaluated the stability of test emulsions under exposure to sunlight, at room temperature (23°C), in cold storage at 4°C, and heated storage at 37°C and 45°C. The most significant results were obtained under the exposure conditions of 37°C and 45°C, where formulations N3, C6, C8 and A3 were unstable after 30 days (see Table 3). However, reviewing the results of their zeta potential and particle size (number and volume) measurements, as Table 2 shows, would have predicted stability changes in the emulsions N3, A3, C6 and C8 shortly after their development. For example, in cationic emulsions, the zeta potential should be positive; and anionic emulsions, negative. Regular stability testing made these changes apparent only after 30 days. The layer structure is a function of the composition and concentration of the surfactant (emulsifier) present. Droplet size is an important factor for emulsion stability, as are the surface charge (zeta potential) of the droplet and interfacial free energy interactions. Thus, the surfactant’s presence serves several functions.18
Combined Approach
In the present work, measuring the zeta potential of emulsions, in addition to other techniques, was important for anticipating possible instabilities, which subsequently were observed in accelerated stability testing. Similar results were obtained by Fairhurst, Dukhin and Klein in preliminary studies on a number of cosmetic emulsions, and these researchers also suggested combining the assessment of particle size distribution and zeta potential for characterizing the stability and performance of cosmetic emulsions.26 The interpretation of their results was equally important in drawing attention to the fact that high zeta in-module potentials > 30 mV are critical for the physicochemical stability of an emulsion, since repulsive forces tend to prevent possible instabilities.27, 28
It should be noted that zeta potential values constitute limited information; however, changes caused in zeta potential values by modifications in the constitution and characteristics of the emulsion represent significant information about the conditions of the interface and, therefore, of the globules in the emulsion.27, 29, 30 It is also important to emphasize that some factors that lower zeta potential, such as low pH values and the presence of electrolytes, may lead to instability and the consequent formation of large oil droplets.31
Conclusion
The results of this study made it possible to help predict the stability behavior of emulsions during the product development stage by analyzing their zeta potential, particle size and distribution. This approach could reduce development times, as these parameters can signal the occurrence of instability in an emulsion immediately after its development and before accelerated stability testing. Further, it can allow stricter production controls to deal with factors that may cause instability in emulsions. As a reminder, however, these analyses should not be used in place of regular accelerated stability testing. Finally, readers are reminded that more experimental evidence is necessary to understand the sensitivity of these methods.
References
- L Lachman, HA Lieberman and JL Kanig, The Theory and Practice of Industrial Pharmacy, Calouste Gulbenkian, LX PT (1986)
- ID Morrison and S Ross, Emulsions: Colloidal Dispersions: Suspensions, Emulsions and Foams, John Wiley & Sons Ltd, NY USA (2002)
- P Fenandez, V André, J Rieger and A Kühmle, Nano-emulsion formation by emulsion phase inversion, Colloids Surf A 251 53–58 (2004)
- S Sajjadi, M Zefra and BW Brooks, Phase inversion in p-xylene-water emulsions with the non-ionic surfactant pair sorbitan monolaurate/polyoxyethylene sorbitan monolaurate (Span20/Tween20), Colloids Surf A 218 241–254 (2003)
- HC Ansel, LV Allen and NG Popovich, Pharmaceutical Dosage Forms and Drug Delivery Systems, Lippincott-Williams and Wilkins, Philadelphia USA (1999)
- MM Breuer, Cosmetics emulsions, in: Encyclopedia of Emulsion Technology, Marcel Dekker, NY (1985) pp 385–424
- M Rieger, Harry’s Cosmeticology, Martin, Chemical Publishing, NY USA (2000)
- LFPD Leon, Stability study of cosmetic products, Cosm & Toil Brasil 13 (4) 54–62 (2001)
- HA Lieberman, MM Rieger and GS Banke, Pharmaceutical Dosage Forms: Disperse Systems, Marcel Dekker, New York USA (1989)
- L Andreas and H Lutz, Evaluation of immobilized enzymes for industrial applications, Chem Soc Rev 42 6236–6249 (2012)
- DJ McClements, Emulsion Stability, Food Emulsions, Principles, Practices and Techniques, CRC Press, Boca Raton, FL USA (2005)
- BF Graham, EF May and RD Trengove, Emulsion inhibiting components in crude oils, Energy Fuels 22 1093–1099 (2008)
- EC Silva and IC Soares, Emulsion technology, Cosm & Toil Brasil 8(5) 37-46 (1996)
- GL Cramp, AM Docking, S Ghosh and J Coupland, On the stability of oil-in-water emulsions to freezing, Food Hydrocolloids 18 899–905 (2004)
- Stability guide for cosmetic products, National Health Surveillance Agency, Federal District of Brazil (2004)
- T Tadros, Application of rheology for assessment and prediction of the long-term physical stability of emulsions, Adv Colloid Interf Sci 108–109 227–258 (2004)
- P Fischer, A Eugster, EK Windhab and M Schuleit, Predictive stress tests to study the influence of processing procedures on long term stability of supersaturated pharmaceutical o/w creams, Intl J Pharmaceutics 339(1–2) 189–196 (2007)
- A Wiacek and E Chilbowski, Zeta potential, effective diameter and multimodal size distribution in oil/water emulsion, Department of Physical Chemistry (1999)
- JM Morais, O Santos and T Delicato, PRF Characterization and evaluation of electrolyte influence on canola oil/water nanoemulsion, J Dispersion Sci Technol 27 1009–1014 (2006)
- L Ursica, D Tita, I Palici, B Tita and V Vlaia, Particle size analysis of some water/oil/water multiple emulsions, Faculty of Pharmacy, University of Medicine and Pharmacy (2005)
- S Corcorran, RY Lochhead and T Mckay, Particle-stabilized emul-sions: A brief overview, Cosm & Toil 119(8) 47–52 (2004)
- DJ Burgess, E Duffy, F Etzler and A Hickey, Particle size analysis: AAPS workshop report, co-sponsored by the U.S. Food and Drug Administration and the U.S. Pharmacopeia, AAPS Journal 6(3) 20 (2004)
- T Deluca, M Kaszuba and K Mattison, Optimizing silicone emulsion stability using zeta potential, reprinted from American Laboratory News (2006)
- R Marsalek, Particle size and zeta potential of ZnO, APCBEE Procedia 9 13–17 (2014)
- I Capek, Degradation of kinetically stable o/w emulsions, Adv Colloid Interface Sci 107 125–155 (2004)
- D Fairhurst, A Dukhin and K Klein, A new way to characterize stability and performance of cosmetic emulsions and suspensions, Dispersion Technology, INC (2001)
- HA Lieberman, MM Rieger and GS Banker, Theory of Emulsions—Pharmaceutical Dosage Forms: Disperse Systems, Marcel Dekker, NY (1998)
- I Roland, G Piel, L Delattre and B Evrard, Systematic characterization of oil-in-water emulsions for formulation design, Int J Pharm 263 (2003)
- JS Stachurski and M Michalek, The effect of the zeta potential on the stability of a non-polar oil-water-oil emulsion, J Colloid Interface Sci 184 (1996)
- Y Gu and D Li, An electrical suspension method for measuring the electric charge on small silicone oil droplets dispersed in aqueous solution, J Colloid Interface Sci 195 (1997)
- C Washington, JA Ferguson and SE Irwi, Computational prediction of the stability of lipid emulsions in total nutrient admixtures, J Pharm Sci 82 808–812 (1993)