
The present work sought to develop in vitro models to study the mechanisms of key hallmarks of aging: cellular communication, senescence and immunoevasion. The models were then used to test the efficacy of natural actives targeting these mechanisms.
Aging is psychosocially and biologically defined as being older. It is a complex and progressive deterioration of physiological functions. The skin, as the largest organ of the body at the interface with the environment, is submitted to intrinsic and extrinsic factors inducing its aging. Aging biology research focuses on understanding both the molecular and cellular mechanisms that underlie these changes.1
In this context, scientific publications identify and categorize the hallmarks of aging.2, 3 The quest for longevity aims to delay the aging process to maintain homeostasis and functionalities of the organism. Among the hallmarks of aging, altered intercellular communication and cell senescence are important mechanisms to investigate. Moreover, the family of sirtuins (NAD+-dependent protein deacylases) is a key actor involved in various biological pathways supporting longevity.
The current work sought to develop in vitro models to study these key mechanisms. It then tested the efficacy of natural actives to target them.
Cell Communication, Senescence and Immunoevasion
To explain how the models were designed, it is important to highlight some of the relevant aspects of skin structure and cell mechanisms underlying skin aging. Briefly, the organization of the dermis affects the firmness of skin, and aging has been proven to disrupt this organization, thus leading to skin slackening.
On the other hand, the epidermis and its properties are not as directly associated with the phenomenon of aging. Indeed, this skin compartment is, above all, considered as the envelope of the body exposed to external aggressions. With aging, it is characterized by a reduced thickness and an alteration of its self-renewal capacity, resulting in a loss of complexion radiance and a deterioration of cutaneous microrelief.
In a tissue as heterogeneous and complex as the skin, dialogue between the different cell types composing it is indispensable for guaranteeing its development and maintaining its biological functions.4-6 The compartments of the dermis and the epidermis cannot be reduced to two separate and autonomous entities. In fact, for several years, studies have been analyzing the influence of the dermis on the aging of the epidermis, particularly by studying mechanisms of intercellular communication.7, 8
In addition, per McHugh and Gil (2018), cell senescence is now described as a central factor of tissue aging. Indeed, all cells possess an intrinsic capital of divisions that when reached, causes the onset of cell senescence. This is a state in which the senescent fibroblast can no longer divide but nevertheless remains metabolically active and therefore capable of affecting the fate of the tissue. Indeed, the fibroblast secretes a large quantity of inflammatory molecules collectively referred to as SASP (senescence-associated secretory phenotype). This SASP alters the functioning of surrounding cells in the dermis and epidermis, thus leading to progressive skin aging.9, 10
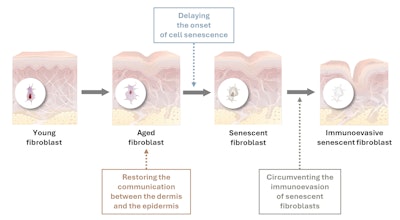
In young skin, immune cells are attracted by senescent cells via their SASP, and after specific recognition, they eliminate them. During aging, however, some senescent fibroblasts install clever strategies dedicated to breaking communication with immune cells and thereby escaping their fatal destiny. This is called the immunoevasion mechanism.
Even though they account for only a small fraction of senescent cells in the dermis, immunoevasive senescent fibroblasts are by far the most harmful cells for the skin. Since they in fact are no longer recognized by the immune system, they are not eliminated, and this elusive population can easily propagate harmful signals in the form of the prolonged secretion of inflammatory molecules composing the SASP.11
The focus of the present work thus focused on the development of three in vitro models of these mechanisms: dermal-epidermal communication, the onset of cellular senescence and the immunoevasion of senescent fibroblasts (see Figure 1). These models were then used to test the ability of given natural actives to target these mechanisms.
Model: Dermal-epidermal Communication
The first model investigated communication from the dermis to the epidermis during aging and its impact on epidermal biological functions. It involved the secretome of human fibroblasts young and aged by successive passages (> P20). Moreover, human keratinocytes from young (< 30 years old) and old donors (> 60 years old) were treated (or not) with a secretome of aged fibroblasts.
The fibroblasts’ secretomes were sent to the Bordeaux Proteome scientific platform for proteomic analysis. The proteome was determined by LC-MS/MS (nano-LC coupled to an Orbitrap Fusion Lumos mass spectrometera) using a quantitative shotgun label-free approach. Proteins that were modulated between young and aged secretomes were analyzed using databases (Reactome, Wikipathways, etc.) to detect biological pathways of interest.
Moreover, extracellular vesicles secreted by fibroblasts were isolated from the secretomeb and characterizedc. The miRNAs in extracellular vesicles were extracted, reverse-transcripted and the complementary DNAs obtained were analyzed by quantitative PCR. Analysis of Ct (relative quantification) was performed using softwared.
Finally, the effects of aged fibroblasts’ secretome on keratinocytes functionalities were investigated thanks to the synthesis of the proliferation marker Ki-67, determined by immunocytofluorescence and the expression of epidermal markers filaggrin, desmoglein-1, aquaporin-3 and laminin-332 by quantitative PCR.
Test ingredient: The effects of a natural Pichia ferment lysate extract were then evaluated on this model.
Results: Dermal-epidermal Communication and Pichia Ferment Lysate Extract
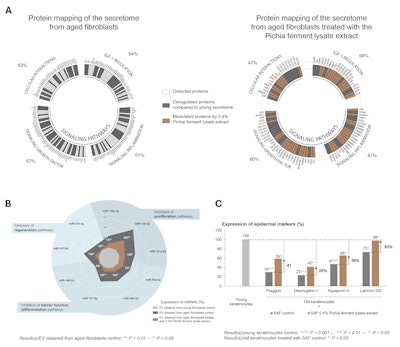
The proteomic analysis of the secretome of young fibroblasts showed that almost 40% of secreted proteins were related to intercellular communication functions. The investigation of these proteins revealed four major biological pathways involved: IGF-1 pathway regulation, inflammation, growth factors and cell interactions.
Compared with young fibroblasts, the secretome of aged fibroblasts was characterized by a modification of the synthesis of more than half the proteins involved in intercellular communication (see Figure 2a), translating to a general degradation of these mechanisms. However, 0.4% Pichia ferment lysate extract regulated the synthesis of proteins involved in different dermis-epidermis communication pathways in the aged fibroblasts (see Figure 2a).
In addition, compared with young fibroblasts, the secretome of aged fibroblasts was characterized by a significant increase in the expression of 9 miRNAs – epigenetic actors transported by extracellular vesicles and described in publications as being involved in regulation of epidermal physiology.12-16 Tested at 0.4% in aged fibroblasts, the Pichia ferment lysate extract significantly limited the expression of miRNAs involved in aging of the epidermis (see Figure 2b).
Finally, it was proven that the secretome of aged fibroblasts had a significant and negative effect on the biological pathways of aged human keratinocytes. However, the secretome of aged human fibroblasts treated with the natural active ingredient at 0.4% applied to aged human keratinocytes significantly improved the synthesis or the expression of Ki-67 by 21%, filaggrin by 41%, desmoglein-1 by 25%, aquaporin-3 by 36% and laminin-332 by 93% (see Figure 2c).
Hence, the Pichia ferment lysate extract restored the dermis-epidermis communication to compensate for the communication shortfall caused by aging, thus favoring epidermal functions. These effects were observed in vivo as well, in a mature Caucasian panel, expressed as the improved renewal and thickness of the epidermis. After 28 days of twice daily use of the extract at 1.7%, panelists’ microreliefs were smoother and skin’s hydration and radiance was restored (not shown).
Model: Delaying the Onset of Cell Senescence
Another model investigated the impact of sirtuins on the dermis during aging, as these have been identified as strategic targets to improve longevity. To this end, human fibroblasts were treated (or not) with a solution of hydrogen peroxide (H2O2) to induce senescence. Then, two sirtuins described for their capacity to limit glycation and delay the onset of cell senescence, as well as their coactivators (NAD+ and phosphorylated AMPK), were investigated.17, 18
Cells were recovered for the luminescence assay of intracellular NAD+. The levels of phosphorylated AMPK, of SIRT1 and SIRT7 were quantified by capillary Western blot. The ratio of the quantity of AMPK-P over the total quantity of AMPK was calculated and expressed as arbitrary units (AU). A cell staining kit was used to visualize SA-β-galactosidase activity, a senescence marker. DAPI labeling was used to obtain cell counts and the ratio of the number of positive cells showing β-galactosidase staining near the nucleus. The results were expressed as percentage of SA-β-galactosidase-positive cells.
Test ingredient: The effects of a natural Myrtus communis leaf extract were then evaluated on this model.
Results: Delaying Cell Senescence and Myrtus Communis Leaf Extract
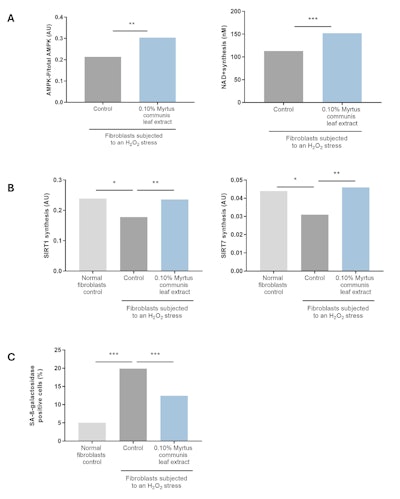
The syntheses of SIRT1 and SIRT7 were significantly reduced with H2O2-induced aging. However, tested at 0.10%, the Myrtus communis leaf extract significantly increased the phosphorylation of AMPK and the production of NAD+ by 42% and 35%, respectively (see Figure 3a). The extract also significantly restored the syntheses of SIRT1 and SIRT7 by 95% and 115%, respectively (see Figure 3b).
In response to senescence-induced stress, the number of SA-β-galactosidase-positive cells increased significantly. When 0.10% Myrtus communis leaf extract was applied at the same time as the senescence-inducing stress, it significantly limited the number of SA-β-galactosidase-positive cells by 50% (see Figure 3c). By limiting glycation and the onset of fibroblast senescence, this natural active ingredient preserved the networks of collagen I by 39% and elastin by 94% (data not shown).
What’s more, in vivo, after 14 days of treatment with 1% active in a body care formula, Caucasian and Asian volunteers observed firming benefits. After applying the active at 2% for 28 days, subjects’ wrinkles were attenuated wrinkles and the quality of the matrix and biomechanical properties of the skin were improved.
Model: Circumventing Immunoevasion
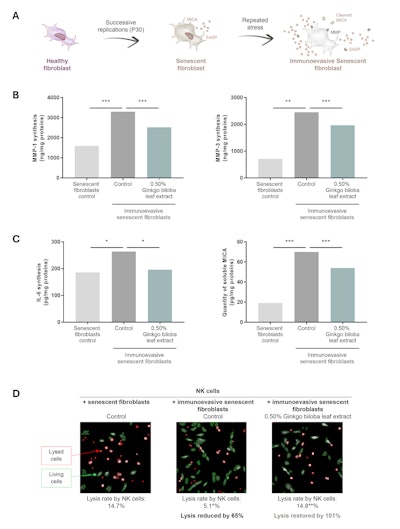
A third in vitro model was developed to assess the impact of immunoevasive senescent fibroblasts on the skin. Senescent fibroblasts were obtained by subjecting normal human fibroblasts to successive replications (P30). The immunoevasive phenotype was then activated by the application of a repeated stress to this senescent model. The SASP composition was determined by the study of the secretions of MMP-1, MMP-3 and IL-6 by ELISA assay. The immunoevasion mechanism was assessed by analyzing the cleavage of the immunogenic “eat me” signal MICA by ELISA assay and the lysis of immunoevasive senescent fibroblasts by Natural Killer (NK) cells by a morphological analysis using a fluorescent probe (see Figure 4a).
Test ingredient: The effects of a natural Ginkgo biloba leaf extract were then evaluated on this model.
Results: Circumventing Immunoevasion and Ginkgo Biloba Leaf Extract
Compared with senescent fibroblasts, immunoevasive senescent fibroblasts secreted significantly more MMP-1, MMP-3 and IL-6. However, 0.5% Gingko biloba leaf extract significantly limited the secretion of deregulated proteins of the SASP by immunoevasive senescent fibroblasts by 46%, 28% and 87%, respectively (see Figure 4b).
In addition, the quantity of MICA cleaved in immunoevasive senescent fibroblasts was significantly higher than that in senescent fibroblasts. Tested at 0.5% on immunoevasive senescent fibroblasts, the Gingko biloba active ingredient significantly limited the release of MICA by 31% (see Figure 4c). It therefore enabled senescent cells to retain their ligand for recognition by the immune system.
Compared with senescent fibroblasts, the lysis of immunoevasive senescent fibroblasts by NK cells was significantly lower. Tested at 0.5% on immunoevasive senescent fibroblasts, the natural active ingredient significantly restored their lysis by NK cells by 101% (see Figure 4d). It therefore re-established the elimination of these resistant senescent cells by NK cells.
Applied to aged human fibroblasts, the secretome of immunoevasive senescent fibroblasts pretreated with the Ginkgo biloba leaf extract at 0.5% significantly preserved the collagen I network synthesis by 64%. The Ginkgo biloba leaf extract therefore preserved the matrix environment by regulating the SASP composition secreted by immunoevasive senescent fibroblasts.
What’s more, after 28 days of treatment in mature Caucasian skin, the natural active ingredient improved the density of the matrix and elasticity, attenuated wrinkles and enhanced complexion radiance (not shown).
Conclusion
As shown here, in vitro models mimicking particular hallmarks of skin aging were successfully developed and used to substantiate the efficacy and mechanisms of three natural actives against these skin aging factors. Hence, restoring the communication between the dermis and epidermis, delaying the onset of cell senescence, and reactivating the immune system’s natural elimination of senescent fibroblasts are demonstrated as three innovative strategies to improve skin longevity.
Footnotes
a Thermo-Fisher
b exoEasy Maxi Kit, Qiagen
c NanoSight LM14, Malvern
d LC480 software, Roche
References
1. Kumar Singh, S., Li Lin, C. and Kumar Mishra, S., eds. (2022). The aging: Introduction, theories, principles and future prospective. In: Anti-aging Drug Discovery on the Basis of Hallmarks of Aging. Academic Press, pp 1-17; available at https://www.amazon.com/Anti-Aging-Discovery-Basis-Hallmarks-Aging/dp/0323902359
2. López-Otín, C., Blasco, M.A., … Partridge, L., et al. (2023). Hallmarks of aging: An expanding universe. Cell 186 243-278.
3. López-Otín, C., Blasco, M.A., ... Partridge, L., et al. (2013). The hallmarks of aging. Cell, 153 1194-1217.
4. Quan, C., Cho, M.K., … Shao, Y., et al. (2015). Dermal fibroblast expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal keratinocyte proliferation in normal and diseased skin. Protein Cell 6 890-903.
5. Wang, Z., Wang, Y., … Farhangfar, F., et al. (2012). Enhanced keratinocyte proliferation and migration in co-culture with fibroblasts. PloS One 7 e40951.
6. Jevtić, M., Löwa, A., … Nováčková, A., et al. (2020). Impact of intercellular crosstalk between epidermal keratinocytes and dermal fibroblasts on skin homeostasis. Biochim Biophys Acta Mol Cell Res 1867 118722.
7. Gruber, F., Kremslehner, C., … Eckhart, L., et al. (2020). Cell aging and cellular senescence in skin aging — Recent advances in fibroblast and keratinocyte biology. Exp Gerontol 130 110780.
8. Wlaschek, M., Maity, P., ... Makrantonaki, E., et al. (2021). Connective tissue and fibroblast senescence in skin aging. J Invest Dermatol 141 985-992.
9. Scudellari, M. (2017). To stay young, kill zombie cells. Nature 550 448-450.
10. Pils, V., Ring, N., … Valdivieso, K., et al. (2021). Promises and challenges of senolytics in skin regeneration, pathology and aging. Mech Aging Dev 200 111588.
11. Ezure, T., Sugahara, M., and Amano, S. (2019). Senescent dermal fibroblasts negatively influence fibroblast extracellular matrix-related gene expression partly via secretion of complement factor D. BioFactors Oxf Engl 45 556-562.
12. Chevalier, F.P., Rorteau, J., …Ferraro, S., et al. (2022). MiR-30a-5p alters epidermal terminal differentiation during aging by regulating BNIP3L/NIX-dependent mitophagy. Cells 11 836.
13. Vaher, H., Runnel, T., … Urgard, E, et al. (2019). MiR-10a-5p is increased in atopic dermatitis and has capacity to inhibit keratinocyte proliferation. Allergy 74 2146-2156.
14. Terlecki-Zaniewicz, L., Pils, V., … Bobbili, M.R., et al. (2019). Extracellular vesicles in human skin: Cross-talk from senescent fibroblasts to keratinocytes by miRNAs. J Invest Dermatol 139 2425-2436.e5.
15. Toury, L., Frankel, D., … Airault, C., et al. (2022). MiR-140-5p and miR-140-3p: Key actors in aging-related diseases? Int J Mol Sci 23 11439.
16. Gerasymchuk, M., Cherkasova, V., … Kovalchuk, O., et al. (2020). The role of microRNAs in organismal and skin aging. Int J Mol Sci 21 5281.
17. Huang, K., Huang, J., … Xie, X., et al. (2013). Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med 65 528-540.
18. Samoilova, E.M., Romanov, S.E., ... Chudakova, D.A., et al. (2024). Role of sirtuins in epigenetic regulation and aging control. Vavilov J Genet Breed 28 215-227.







!['We believe [Byome Derma] will redefine how products are tested, recommended and marketed, moving the industry away from intuition or influence, toward evidence-based personalization.' Pictured: Byome Labs Team](https://img.cosmeticsandtoiletries.com/mindful/allured/workspaces/default/uploads/2025/08/byome-labs-group-photo.AKivj2669s.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&fp-x=0.49&fp-y=0.5&fp-z=1&h=191&q=70&w=340)



