* Reprinted from H Zhai, S Barbadillo, X Hui and HI Maibach, In vitro model for decontamination of human skin: Formaldehyde, Food and Chemical Toxicology 45(4) 618-21; with permission from Elsevier Ltd. (Copyright 2007).
Decontamination of a chemical from skin is often an emergency measure. The present study utilized an in vitro model to compare the decontamination capacity of three model decontaminant solutions: tap water, isotonic saline and hypertonic saline. Human cadaver skin samples were dosed with radio-labeled [14C]-formaldehyde and the surface skin of each sample was washed after each exposure with one of the three model decontaminant solutions.
After washing, the skin was stripped with tape discs and the wash solutions, strippings, receptor fluid and remainder of skin were counted to measure the amount of formaldehyde present. An evaporation test was also conducted to monitor the percentage of formaldehyde evaporation.
The percentage of formaldehyde evaporation increased linearly with extending application times and the data suggests that isotonic saline may be effective in removing formaldehyde from skin, as the authors show here. This model may accelerate researchers’ knowledge of decontamination mechanisms and thus lead to enhanced efficacy.
Background
Chemical injuries are commonly encountered following exposure to acids and alkali, including hydrofluoric acid, formic acid, anhydrous ammonia, cement and phenol. The concentration of corrosive agents, and potency and duration of their contact primarily determine the degree of skin destruction. Tregear1 initiated this field in the 1940s, but practical interventions have since remained limited.
Immediately following exposure to such chemicals, washing with water, or soap and water, is the traditional measure to reduce damage and minimize percutaneous penetration. Wester et al.2 reported that the removal of alachlor with water alone was less effective than doing so with soap and water. A traditional soap and water wash and the emergency water shower are relatively ineffective at removing methylene bisphenyl isocyanate, a potent contact sensitizer, from skin.3 Thus, water or soap and water may not be the most effective means of skin decontamination, particularly for lipophilic materials. In some cases, the amount of chemical that remains on the skin after traditional washing procedures can have toxic consequences.4 The need for further development of robust decontamination agents thus becomes apparent.
Formaldehyde, used widely by various industries, is a common allergen and irritant5 and was thus selected as a model compound. This study compared the capacity of decontamination solutions utilizing an in vitro model system on human skin.
Materials and Methods
Contaminant: An aqueous solution of radio-labeled [14C]-formaldehydea (0.1 mCi/mL; specific activity: 51.9 mCi/m mol) was used for this study.
Model decontamination solutions: Isotonic salineb (0.9%; pH = 5.94) and hypertonic salineb (1.8%; pH = 5.71) also were obtained for the present study. The tap water used (pH = 8.09) was taken from the faucet, the source of which was the Hetch Hetchy reservoir, located in California’s Sierra Nevada Mountains.
Human skin: Human cadaver skinc was dermatomed to a 500-µm thickness. Skin samples were stored in Eagle’s Minimum Essential Mediad and refrigerated at 4°C prior to use within five days after death to maintain viability.6–8 A total of five skin samples were used.
Procedure: Skin was placed onto glass diffusion cells with rubber bands. The diffusion cells had been pre-filled with a maximum amount of the receptor fluid: 0.9% sodium chloride, approximately 6 mL in each. Then, aliquots 10 µl (approximately 0.25 µg) of [14C]-formaldehyde solution was dosed by a high performance liquid chromatography syringe onto each skin surface. After exposure intervals of 1 min, 3 min, and 30 min post-dosing, the surface skin (3 cm2) was washed with 4 mL of each solution per time; thus, a total of 12 mL of each solution was used to wash off one sample. All washing liquids were collected individually into a scintillation glass vial for radioactive measurement. The skin was then stripped twice with tape discse, superficially removing chemical residue from the skin. Lastly, the remaining amounts of [14C]-formaldehyde in the wash solutions, strippings, receptor fluid and remaining skin were determined.
Evaporation test and scintillation counting: Evaporation tests monitored the percentage of [14C]-formaldehyde evaporation at exposure times of 1 min, 3 min, 15 min, 30 min and 60 min. Plastic discs, 1.75 cm in diameter and 0.178 mm thick, were each applied with 1 µCi/10 µL/cm2 of [14C]-formaldehyde and timed for the appropriate duration of exposure. Triplicates for each time point were taken, yielding a total of 15 disc samples, after which each disc sample was immediately placed into its designated scintillation vial; the vial was then filled with a pre-designated liquid compoundf.
Background control samples and the test samples were counted in a computer-controlled liquid scintillation analyzerg. Control and test sample counts were then transferred to a computer program that subtracted background control samples and generated a spreadsheet data report. The in-house counting process and the computer program were verified by a quality assurance officer.
Statistical analysis: Statistical analysis was performed utilizing a computer programh. Differences were analyzed utilizing the One Way Repeated Measures ANOVA. Statistical significance was accepted at p < 0.05.
Results
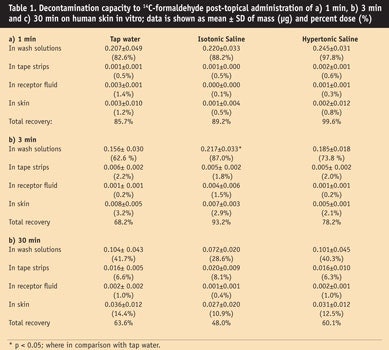
The mean values, standard deviations (SD) and percent dose of [14C]-formaldehyde obtained from each group are summarized in Table 1. No statistical decontaminating differences were found among those groups, except in the case of isotonic saline at 3 min post-exposure in wash solutions, which showed a significant difference (87%, p < 0.05) when compared with tap water (62.6%) (see Table 1b and Figure 1). The percentage of formaldehyde evaporation was found to increase linearly (R2 = 0.94) with extended application times (see Figure 2). Evaporation = 10.8 + 0.433 time; the percentage doses of evaporation of formaldehyde were, respectively: 7.7%, 13.6%, 19.7%, 24.4% and 35.9% at correlating time intervals of 1 min, 3 min, 15 min, 30 min and 60 min.
Discussion
A previous study2 indicated that skin decontamination of alachlor at 0 hr with soap and water removed 73% ± 15.8% (n = 4) of the applied dose with the first wash, and that the amount removed increased to 82.3% ± 14.8% with two additional washes. Decontamination after 1 hr was found to remove 87.5% ± 12.4% with three successive washes. After 3 hr the decontamination ability decreased, and after 24 hr only 51.9% ± 12.2% could be recovered with three successive washes. Using water only, at 0 hr, 36.6% ± 12.3% alachlor was removed with the first wash and the total increased to 56.0% ± 14.0% with two additional washes. And at 24 hr, the total amount decreased to 28.7% ± 12.2% for three successive washes. Continual successive washes (6–8, in sequence) recovered 80–90% of the skin-applied alachlor.
In the current study, at all time points, most formaldehyde was recovered in the wash solutions. By the 3-min mark, only isotonic saline provided statistically significant enhanced decontamination, compared with tap water. By 30 min, significance was lost. Results indicated that, overall, the three model decontamination solutions were almost equally effective in removing the applied dose of formaldehyde. However, isotonic saline provided a slight enhancement.
The volatility of the dose applied of formaldehyde to skin was determined and showed a linear trend with extending application time. Even with the rapid formaldehyde evaporation, penetration into skin occurred within 30 min post-application. As noted, obtaining total mass balance proved difficult, presumably due to volatility. Mass balance accountability is 48–99.6%, compared with the previous in vivo study with a less volatile chemical in which dose accountability was 80.6–95.2%.2 Thus, a less volatile contaminant such as an irritant and/or allergen could simplify model development. Now that this practicable model has been developed, a factorial design approach can be utilized to improve decontamination methodology.
The present authors had previously developed a high throughput model utilizing ground callus and delipidized callus, noting a relationship of binding to callus and penetration of applied chemicals.9, 10 Relating the callus assay to the current model may be of value since both in vitro methods provide a system that might aid in the prediction of decontamination assessment.
Based on the solubility of variables such as: contaminant and decontamination solutions, pH, the volume of decontamination, time of removal ratios, and physical enhancements (e.g., stripping, rubbing, adhesives; defining binding properties); robust decontamination agents/systems may be developable. For instance, a recent controlled experiment showed that water rinsing followed by topical calcium provided favorable results for hydrofluoric acid skin decontamination.11 Note, however, details provided by Hall et al.12 that may have significantly influenced the results; for example, the immediate use of a skin decontamination agent as a critical factor of efficacy following hydrofluoric acid exposure. In addition, adoption of splashing or acute burning models may also dramatically result in diametrically opposite outcomes with regard to decontamination agent potency. Wester et al.13 summarize the literature on skin decontamination, and Moody and Maibach14 the “wash-in” effect, or water-enhancing penetration.
Conclusion
The present model could be used as a rapid screening procedure for the development of effective decontamination agents. Taken together, the authors do not wish to overgeneralize these results. Yet, the in vitro human skin model may—when validated with other chemicals and subsequent in vivo verification2, 3—provide more rapid enhancement of practical knowledge in this complex field.
Reproduction of all or part of this article strictly is prohibited.
References
1. RT Tregear, ed, Physical functions of skin, London: Academic Press (1966)
2. RC Wester, J Melendres and HI Maibach, In vivo percutaneous absorption and skin decontamination of alachlor in rhesus monkey, J Toxicol Environ Health 36 1–12 (1992)
3. RC Wester, X Hui, T Landry and HI Maibach, In vivo skin decontamination of methylene bisphenyl isocyanate (MDI): Soap and water ineffective compared to polypropylene glycol, polyglycol-based cleanser, and corn oil, Toxicol Sci 48 1–4 (1999)
4. P Kintz, A Tracqui and P Mangin, Accidental death caused by the absorption of 2,4-dichlorophenol through the skin, Arch Toxicol 66 298–299 (1992)
5. MD Pratt et al, North American Contact Dermatitis Group patch-test results, 2001–2002 study period, Dermatitis 15 176–183 (2004)
6. LN Hurst, DH Brown and KA Murray, Prolonged life and improved quality for stored skin grafts, Pla Reconstr Surg 73 105–109 (1984)
7. RL Bronaugh, RF Stewart and JE Storm, Extent of cutaneous metabolism during percutaneous absorption of xenobiotics, Toxicol Appl Pharmacol 99 534–543 (1989)
8. RC Wester, J Christoffel, T Hartway, N Poblete and HI Maibach, Cadaver human skin viability for in vitro percutaneous absorption: storage and detrimental effects of heat-separation and freezing, Pharm Res 15 82–84 (1998)
9. X Hui, RC Wester, PS Magee and HI Maibach, Partitioning of chemicals from water into powdered human stratum corneum (Callus): A model study, In Vitro Toxicology 8 150–167 (1995)
10. RC Wester et al, Polymers effect on estradiol partition coefficient between powdered human stratum corneum and water, J Pharm Sci 91 2642–2645 (2002)
11. J Hojer, M Personne, P Hulten, U Ludwigs, Topical treatments for hydrofluoric acid burns: A blind controlled experimental study, J Toxicol Clin Toxicol 40 861–866 (2002)
12. AH Hall, J Blomet and L Mathieu, Topical treatments for hydrofluoric acid burns: A blind controlled experimental study (letter), J Toxicol Clin Toxicol 41 1031–1032 (2003)
13. RC Wester and HI Maibach, Pesticide percutaneous absorption and decontamination, in Handbook of Pesticide Toxicology, Principles, 2nd edn, RI Krieger, ed, San Diego: Academic Press (2001) pp 905–912
14. RP Moody and HI Maibach, Skin decontamination: importance of the wash-in effect, Food Chem Toxicol 44 1783–1788 (2006)





!['We believe [Byome Derma] will redefine how products are tested, recommended and marketed, moving the industry away from intuition or influence, toward evidence-based personalization.' Pictured: Byome Labs Team](https://img.cosmeticsandtoiletries.com/mindful/allured/workspaces/default/uploads/2025/08/byome-labs-group-photo.AKivj2669s.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&fp-x=0.49&fp-y=0.5&fp-z=1&h=191&q=70&w=340)




