Corneocyte maturity plays a crucial role in dermatology and cosmetic science because immature corneocytes in the stratum corneum (SC) can cause impaired barrier properties. This poses a threat to overall health, since it is the outermost layers of the SC that protect the body from invasion by pathogens and viruses.1, 4 Interestingly, studying corneocyte maturity has enabled dermatologists to determine there are no differences in corneocyte maturity across different racial groups. Further, a causal relationship has been found between excessive amounts of immature corneocytes and dry skin in all ethnic groups.2
Since corneocytes act as indicators, they can be used to correlate the age and health of skin. Therefore, the techniques discussed here could be used to determine the efficacy of skin care products. For example, cosmetic scientists have demonstrated that the use of cosmetic moisturizers containing niacinamide and hexamidine help promote the development of mature corneocytes, in turn improving the barrier properties of skin.3 This enhancement of barrier properties is attributed to increased levels of tortuosity and better covalent bonding of the SC to intercellular lipids that protect skin and prevent water loss.
Such barrier properties are localized in the topmost layer of the skin, i.e., the SC, which is a two-component system of corneocytes embedded in a lipid matrix. Corneocytes consist of a stabilized array of protein (keratin) filaments contained within a covalently crosslinked cornified envelope (CE). The deeper layers of the SC contain irregular shaped CEs that are very fragile. Mature corneocytes found in the outermost layers of the SC are highly cross-linked and extremely hydrophobic compared to the immature corneocytes found deeper in the SC. This cross-linking occurs in precursor proteins assisted by transglutaminase enzymes.1, 4
The formation of the CE assembly provides the foundation for the barrier function of skin and is an extremely complex process.1 Briefly, a key biochemical pathway involves omega-hydroxylation of ceramides by cytochrome P450. Assembly of the hydrophobic constituents of the CE includes intercellular lamellar lipids consisting primarily of cholesterol, ceramides and free fatty acids that become organized to contribute to the barrier function of the SC. The importance of studying corneocytes is clear when one considers corneocytes as being correlated with the age and health of skin.1, 4, 5
Analytical Techniques
Traditional analytical techniques used to study skin include: scanning laser microscopy, confocal Raman microscopy, microfluorometry, electron microscopy and ultrasound microscopy. 6 The purpose of the present study was to compare a well-known microfluorometry technique to a novel method based on atomic force microscopy (AFM) to distinguish mature from immature corneocytes. Microfluorometry involves determining the fluorescent intensity of corneocytes after treatment with the lipophilic dye Nile red. It is well-known that mature corneocytes are cross-linked, which imparts lipophilic properties; these properties react strongly with the dye.
The AFM method in contact mode generates force-distance (F-D) curves by using the probe tip to first approach and then retract from the corneocyte surface, thus creating a hysteresis loop. The tip/corneocyte interaction reflects the mechanical properties of the cell, and since cross-linked corneocytes are stiffer than immature corneocytes, the adhesive energy represented by the area under the hysteresis loop is expected to be greater in mature corneocytes than immature corneocytes.
Microfluorometry and Epifluorescence
Fluorescence microscopy utilizes a high intensity light such as a mercury lamp to illuminate samples. This light excites fluorescent species within the sample, which then emit light of a longer wavelength. Unlike conventional microscopes, both the excitation and emission light travel through the objective lens. Therefore, optics are used to separate the original illumination (excitation) light from the fluorescence emission emanating from the sample, forming an image. This optical technique is referred to as epifluorescence. A schematic diagram of the instrument is illustrated in Figure 1. Microfluorometry was performed using such an instrumenta, which operates in the incident mode in the visible range at a specific wavelength, as determined by the filter selected. The related instrument settings used for the study are shown in Table 1.
Atomic Force Microscopy
AFM uses a sharp tip mounted on a cantilever, in this case both composed of single crystal silicon, with a nominal spring constant of 0.02 Newtons per meter (N/m), which has the sensitivity to detect the Van der Waals, electrostatic and capillary surface forces, among others, that interact with it. This assembly enables the generation of three-dimensional topographic images of a surface with subangstrom resolution in the Z direction, i.e., perpendicular to the surface. AFM often is referred to as just one technique of many under the umbrella term of scanning probe microscopy (SPM). In fact, an image can be created from almost any physical property, such as friction, elasticity and electric force, as well as differences in chemical composition. Further, AFM can be performed using three different imaging modes: contact; intermittent contact, i.e., tapping; and non-contact mode. All data generated for the present study was obtained using the contact mode.
Force-distance Curves
In addition to creating 3-D images of the surface of materials, AFM can be utilized in the so-called spectroscopic mode, which is accomplished by generating F-D curves.7, 8 As noted, the amount of force felt by the probe tip is detected by the cantilever as it is brought closer, approaching the sample surface, and then retracted. These two steps create a hysteresis loop that captures the tip/sample interaction. This interaction is affected not only by the morphology of the sample surface, but also by the chemical and physical properties of the sample as well as environmental factors. Although one could also generate F-D curves in the tapping mode, the contact mode was used exclusively since the cantilever can only be calibrated in this mode. A calibrated cantilever makes it possible to obtain quantitative data.
Tracing the path of a typical F-D curve, as the probe tip approaches the sample surface, intermolecular forces are initially sensed until the tip is repulsed by interatomic forces. This is evidenced by the concave shape of the cantilever (see Figure 2a). During tip withdrawal, adhesive surface forces become active and actually drag the probe tip down into the surface, causing the cantilever to bend, forming a convex shape (see Figure 2d). Eventually the tip breaks free from the surface and is no longer influenced by surface forces (see Figure 2b). The Van der Waals force represents just one contribution to the cantilever deflection; other forces include capillary and electrostatic.
Sample Preparation
For the described tests, corneocytes were collected from the ventral surface of the forearm of a healthy 26-year-old male. The topmost layer of skin flakes was first removed utilizing regular adhesive tape. The corneocytes were then collected by tape-stripping the same area 10 times. Since corneocyte maturity and surface area are known to decrease with depth,4 tape-strippings were collected repeatedly from the same site. Thus, the 10th tape represented skin cells from the deepest layer of the SC.9
The corneocyte samples were prepared for microfluorometry following the method utilized by Hirao et al.5 Briefly, the tape containing the corneocytes was cut into small pieces and immersed in 1 mL of dissociation buffer consisting of 2% sodium dodecyl sulfate; added to 20 mM dithiothreitol- 5 mM EDTA and 0.1 M Tris-HCl buffer adjusted to pH 8.5; and boiled at 100°C for 10 min. The corneocytes that debonded from the tape were collected as a precipitate by centrifugation for 10 min. A small quantity of precipitated cells was smeared onto a glass slide using a pipette and allowed to dry. The cells were fixed by placing a few drops of methanol on the glass slide for 1 min and briefly rinsed with deionized water.
After drying, the cells were stained with Nile red dye,10 which as noted fluoresces when it reacts with any lipophilic species. The dye is soluble in lipids and does not dissolve them. For the staining procedure, a 0.5% solution of Nile red was prepared in 75% glycerol, then placed on the glass slide with a pipette containing the corneocytes for 2 min. The stain was rinsed from the slide for 5 sec using deionized water and placed in the dark to dry.
Results and Discussion
Microfluorometry: Before analysis, the spectrometer of the epifluorescence microscope was set to the parameters shown in Table 1. Microfluorometry was then performed after translating the centerable field diaphragm of the microscope directly over the center of the corneocyte by adjusting two screws that correspond to the x and y axes. Fluorescence intensity was determined utilizing a circuit composed of a voltmeter integrated with various electronic modules. The digital readout was obtained using a computer program developed in-house that displays the count rate continuously. A typical epifluorescent image of corneocytes stained with the Nile red is shown in Figure 3. It can be seen that the corneocytes have not stained uniformly; an indication that the degree of cross-linking varied between the different cells.
The microfluorometry results summarized in Table 2 indicate that the mature corneocytes had a higher average fluorescence intensity (54) than the immature corneocytes (43).9 These results support the fact that mature CEs are known to be rigid, insoluble structures; this rigidity results from the cross-linking present in mature corneocytes. A student’s t-test yielded p = 0.04, indicating the fluorescence of the corneocytes was significantly different, at a 95% level of confidence.
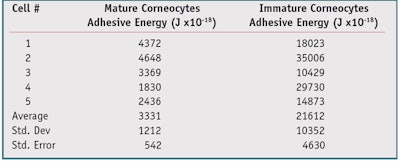
F-D curves: A typical F-D curve for a mature versus an immature corneocyte is shown in Figure 4. Note that the area under the hysteresis loop of the F-D curve represents adhesive energy. To demonstrate the utility of the method, F-D curves were generated from five mature and five immature corneocytes. The data in Table 3 shows that the immature corneocytes had a higher adhesive energy (21,612 Joules x10-18) than the mature corneocytes (3,331 Joules x10-18).
The higher level of adhesive energy observed for immature corneocytes can be understood in terms of contact area. When the AFM tip makes contact with an immature corneocyte, the tip meets little resistance. Since the corneocyte is not cross-linked, it penetrates easily, resulting in a higher level of interfacial contact. The increase in contact relative to the mature, cross-linked (stiffer) corneocyte gives rise to an increase in the adhesive energy due to van der Waals and capillary attractive forces that act between the AFM tip and corneocyte surface. This result was expected since the mature corneocytes, being crosslinked, are stiffer than the immature corneocytes.
Statistical analysis via a student’s t-test gave p = 0.02, indicating the average value for the adhesive energy obtained for the mature corneocytes was significantly different than the value obtained from immature corneocytes, at a 95% confidence level. Although contaminants, lubricants and even a thin layer of water can affect this measurement, which is often present when operating the AFM in air, these effects were determined to be minimal.
AFM images: Three-dimensional AFM images of both mature and immature corneocytes are shown in Figure 5 and Figure 6. The images to the left in both figures show topography, whereas those to the right show deflection error. The X and Y directions of the images were 30 microns each in length. The Z direction extends from the lowest elevation, appearing as the darker areas in the image, to the highest elevation, appearing as the lightest areas. A height scale is shown as a vertical bar to the right of each image. The scale reaches a maximum of 3.9 microns for the topographic image of the mature corneocyte, and 6 microns for the corresponding image of the immature corneocyte.
The cross-linking in a typical mature corneocyte appears ropelike (see Figure 5), in contrast to the smoother surface topography exhibited by an immature corneocyte (see Figure 6). The Z scale in deflection error images are meaningless since they only show how the tip is deflected as it encounters sample topography. Deflection images are generated from the error signal and enhance the outlines of features; they are equivalent to a map showing the slope of a sample’s features. These images often show the shape of an image’s features more clearly than topographic images.
Conclusion
Data collected from the novel F-D assessment method described provided results consistent with microfluorometric data. Both methods were able to differentiate between mature and immature corneocytes, although they are designed to detect different phenomena. The F-D technique depends upon detecting minute differences in the intermolecular forces of the corneocytes when they interact with the AFM tip. Microfluorometry essentially is a chemical method that depends on determining the differences in fluorescent intensity of a lipophilic dye when it reacts with corneocytes.
The techniques discussed could be used to determine the efficacy of skin care products. For example, one study5 utilized microfluorometry and a double-staining method to evaluate the degree of corneocyte cross-linking after four weeks of treatment with a cosmetic moisturizer. In this case, the researchers found an 18% (p = 0.002) increase in fully mature corneocytes and a significant decrease in fully immature corneocytes (p = 0.067), as well as a decrease in “less mature” corneocytes (p = 0.038).
Acknowledgements: The author wishes to thank the following individuals from TRI Princeton: David Moore, for his helpful discussions regarding cross-linking in corneocytes; Joel Coret, for his advice in preparing solutions for preparing corneocytes; Simona Jusyte for her advice on corneocyte fixation techniques; and Samuel Gourion, for his suggestions regarding experimental design and assisting with the microfluorometry. He also thanks Nan Yao, director of Princeton University’s Imaging and Analysis Center (IAC), for providing access to the AFM, and technical specialist Jerry Poirier. Finally, he thanks Anthony Ribaudo II, his son, for donating corneocytes for this study.
References
Send e-mail to [email protected].
- R Marks, The stratum corneum barrier: The final frontier, American Society for Nutritional Sciences 0022–3166/04 (2004)
- AV Rawlings, Ethnic differences in stratum structure and function, Household and Personal Care Today, 1 (2010)
- BB Jarrold, PJ Matts, SC Weitz, JR Kaczvinsky, DJ Whittenbarger and R Osborne, Use of a cosmetic moisturizer promotes corneocyte maturity, Procter & Gamble Beauty & Grooming, Cincinnati, USA, available at pgbeautygroomingscience.com/assets/ files/2010_AAD_11_Jarrold.pdf (Accessed Jan 3, 2013)
- MA Farage, KW Miller and HI Maibach, eds, Textbook of Aging Skin, Springer-Verlag Berlin Heidelberg, 413 (2010)
- M Hirao, M Denda and M Takahashi, Identification of immature cornified envelopes in the barrier-impaired epidermis by characterization of their hydrophobicity and antigenicities of the components, Exper Derm 10 35–44 (2001)
- SJ Lin et al, Investigation of the mechanism of transdermal penetration enhancer a comparison of multiphoton microscopy and electron microscopy, proceedings of the International Society for Optical Engineering (SPIE) vol 6842-684203 (2008)
- RM Gaikwad, SI Vasilyev, S Datta and I Sokolov, Atomic force microscopy characterization of corneocytes—Effect of moisturizer on their topology, rigidity and friction, Skin Res and Tech 16 275–282 (2010)
- C Soussen, D Brie, F Gaboriaud and C Kessler, Modeling of force-volume images in atomic force microscopy, IEEE 978-1-4244- 2003-2 (2008) pp1605–1608
- D Mohammed, PJ Matts, J Hadgraft and ME Lane, Depth profiling of stratum corneum biophysical and molecular properties, Brit J Derm 164 957–965 (2011)
- P Greenspan, EP Mayer and SD Fowler, Nile Red: A selective fluorescent stain for intracellular lipid droplets, J Cell Bio, 100 965–973 (Mar 1985)







!['We believe [Byome Derma] will redefine how products are tested, recommended and marketed, moving the industry away from intuition or influence, toward evidence-based personalization.' Pictured: Byome Labs Team](https://img.cosmeticsandtoiletries.com/mindful/allured/workspaces/default/uploads/2025/08/byome-labs-group-photo.AKivj2669s.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&fp-x=0.49&fp-y=0.5&fp-z=1&h=191&q=70&w=340)




