Pivotal research from the late 1950s continues to impact oral care technology development today. At that time, researchers at Indiana University successfully demonstrated the ability of fluoride to be incorporated into toothpaste and deliver an anticavity, or anticaries, benefit. The first toothpaste proven to be clinically effective against caries was the original Cresta brand toothpaste, a product formulated with stannous fluoride (SnF2) in an abrasive system that was only partially compatible with the fluoride active. Earlier toothpastes had combined ionic sodium fluoride, a highly reactive species, with calcium-based abrasives such as calcium carbonate. The active anticaries agent, the F- ion, reacted with free calcium in these formulations, forming insoluble calcium flouride, which produced clinically ineffective products.1, 2
In the early 1960s, toothpastes formulated with other fluoride actives, sodium monofluorophosphate (SMFP) and amine fluoride were proven to be clinically effective. SMFP, a covalently bound form of fluoride, can be formulated with calcium abrasives since its reactivity is reduced in the formulation. In order to provide an anticavity benefit, SMFP must be hydrolyzed, generally from salivary enzymes, to release free fluoride. Note that amine fluoride, a fluoride/surfactant combination, is not available in the U.S. market.
In the early 1980s, a fluoride-compatible silica system was perfected, enabling the delivery of high levels of bioavailable fluoride in clinically effective formulations. Today, the vast majority of toothpastes sold globally are formulated with one of these four clinically proven fluoride salts (see Figure 1), i.e., sodium fluoride, amine fluoride, SMFP or stannous fluoride.
One major issue with the original stannous fluoride toothpaste from the 1950s was bioavailability of the active; only about 25% of the fluoride was bioavailable, and stannous bioavailability was close to 0%. In the 1990s, a new formula was thus developed with stannous fluoride in a silica abrasive system that delivered higher levels of fluoride and stannous compared to the original formula. As noted by Tinanoff,3 there are three chemical routes suited to improving the stability of SnF2: 1) the use of sacrificial stannous salts as antioxidants, which react with available oxygen and leave stannous fluoride protected; 2) the addition to formulations of stannous salts such as stannous pyrophosphate, which provides a reservoir of stannous to enhance shelf life; and 3) the addition of chelating agents to formulations to protect SnF2 complexes. These approaches were combined and resulted in a formula that was both shelf-stable and therapeutically effective.4
However, the then-available higher level of stannous fluoride had the potential to cause extrinsic tooth staining by driving chemical reactions in the biofilm. Thus, in 2005, the formula was revised again and developed with stabilized stannous fluoride in a silica abrasive system, which provided significantly greater bioavailability of both stannous and fluoride, a marked improvement over the original formulation, without the issue of staining due to the addition of sodium hexametaphosphate in the formulation. Sodium hexametaphosphate has been proven to be a particularly effective whitening agent, countering staining issues related to highly bioavailable SnF2.
Over the past several decades, research5 has focused on improving and expanding the benefits and performance of toothpaste. Although the incorporation of new benefits into fluoride-based toothpastes has not been a simple task, formulators have worked closely with both dental and consumer researchers to ensure the stability and delivery of actives in consumer viable products. Consumer markets have witnessed a proliferation of highly effective oral care products, including anti-tartar, anti-plaque and anti-gingivitis toothpastes, in addition to others designed to provide greater cleaning efficiency and extrinsic tooth whitening for significant therapeutic and cosmetic benefits.
In fact, even cosmetic benefits have proven important as they have fostered better dental hygiene. One of the primary outcomes of better oral health has been that consumers are now retaining their natural teeth well into their senior years, something that was practically unheard of half a century ago. Coupled with this increased tooth longevity, however, comes new issues to tackle; the longer teeth stay in the mouth, the more issues they likely will face, driving the need for new products that are designed to specifically address them.
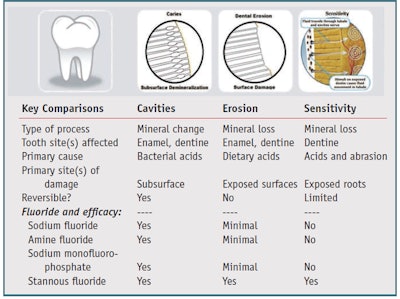
Two oral care issues of current interest are tooth sensitivity and dental erosion. Each of these requires technology advances that go beyond the basics of cavity prevention. While anticavity toothpastes are designed to protect teeth against the progression of cavities, tooth sensitivity and dental erosion are the result of completely different processes that occur on exposed tooth surfaces (see Table 1). Treatments therefore must be designed to specifically target the causative factors for each problem. In order to fully appreciate each of these issues, it is important to understand how each problem occurs.
Tooth Sensitivity
Tooth sensitivity, or dentine hypersensitivity, is the sensation felt when nerves inside the teeth become exposed. Exposed tooth roots often occur as a result of gingival recession and certain toothwear processes, such as dental erosion. 6, 7 Tooth roots are covered with a relatively soft layer of protective mineral called cementum. Cementum is easily softened by dietary acids and is susceptible to removal through abrasive wear. Once cementum is removed, pathways directly connecting to the nerve center of the tooth become opened, putting the tooth at risk of developing sensitivity. The sensation of tooth sensitivity can range from irritation to intense pain. Data suggests the prevalence of tooth sensitivity may be increasing in the general population.8, 9
Dentine contains thousands of microscopic tubules, each approximately 0.5–2.0 mm in diameter. The tubules radiate outward from the pulp of the tooth and contain a plasmalike biological fluid. Changes in the flow of this fluid can trigger mechanoreceptors on nerves located near the pulp, eliciting a pain response. This hydrodynamic flow can be increased by heat, cold, pressure, drying, sugar, sour (dehydrating chemicals) or other forces acting on the tooth.8
Technology Approaches
Although products designed to alleviate tooth sensitivity have been in the market for many years, the efficacy of many of these products has been relatively low. Current efforts aim to enhance the efficacy of sensitivity toothpastes, due in part to the increased consumption of acid-containing beverages that attack and soften the exposed root surfaces, making the cementum easier to remove via mechanical abrasion. One formulation approach for treating hypersensitivity is through delivery of chemical agents that pass through the open dentine tubules and target the pain receptors. Potassium salts such as potassium nitrate have been the most successful and act by reducing the transmission of pain by directly blocking nerve impulses.10 Numerous clinical studies have confirmed the effectiveness of potassium salts for reducing tooth sensitivity.11 One limitation with potassium chemistry, however, is that the sensitivity often takes weeks to subside.
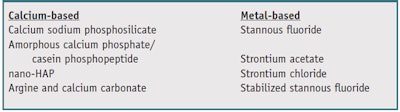
The development of precipitation technologies has been an alternate formulation approach toward reducing tooth sensitivity (see Table 2). This approach aims to occlude the open, exposed dentinal tubules with mineral precipitates capable of withstanding acid and abrasive challenges. Over-thecounter (OTC) toothpaste formulations in this area have taken two paths: calcium-based chemistries designed to simulate natural tooth mineral with precipitates that pack into open tubules; and metal-based chemistries designed to combine with natural tooth minerals to form a more acid-resistant occlusion. Some of the more well-known precipitation approaches delivered from OTC toothpastes include the following.
Casein phosphopepetide, amorphous calcium phosphate (CPP-ACP): Marketed under the trade name Recaldentb, this product was originally designed as a remineralization technology derived from milk protein and intended to deliver calcium and phosphate ions into tooth enamel as either an alternative or supplement to fluoride.
Calcium sodium phosphosilicate: Marketed as NovaMinc, this product is a form of bioactive glass. When contacted with saliva, the active particles release calcium and phosphate ions in an environment that favors the formation of hydroxyapatite—a material similar to natural tooth mineral. The physical occlusion of dentinal tubules with the bioactive glass is the goal of this approach.
Arginine, calcium and bicarbonate: In another approach originally developed as an alternative to fluoride, this combination is currently marketed as a sensitivity treatment under the Pro-Argind trademark. Clinically proven formulations incorporate arginine, an amino acid found in saliva, with calcium carbonate and are designed to rapidly occlude exposed dentinal tubules.
Nano-hydroxyapatite: This nanoparticle approach is intended to enhance the transport of calcium back into teeth by delivering particles of sizes that are better able to penetrate and occlude the open dentinal tubules. Such formulations are marketed under several trade names, including Apagarde, BioRepairf and others; each product uses a different method to develop the nanoparticles employed.
Strontium chloride: Strontium chloride is the active ingredient in the original Sensodyne brandg toothpaste and was designed to precipitate along with calcium and phosphate from saliva into the open dentine tubules, providing a plug that builds up over time, reducing sensitivity after a period of weeks.
Strontium acetate: Similiar to strontium chloride, strontium acetate is an active sensitivity agent that provides an alternative source of strontium. Like strontium chloride, formulations containing strontium acetate are designed to occlude tubules and build a resistant barrier over time.
Stannous fluoride: In addition to its ability to help prevent caries, stannous fluoride has been recognized for years for its potential to help prevent tooth sensitivity through its ability to occlude dentine tubules. However, original formulations were known to stain teeth and did not have long-term stability.
Stabilized stannous fluoride: Similar to stannous fluoride, stabilized stannous fluoride toothpastes were developed to occlude tubules by a combination approach that deposits an acid-resistant stannous-containing precipitate into the open tubules, then coats exposed tooth surfaces with a micro-thin shield to provide prolonged relief. These formulations, marketed under the Crest Pro-Healthh brand, also contain sodium hexametaphosphate to mitigate the issue of stain and provide an added benefit of tooth whitening (see Figure 2).
Dental Erosion
Dental erosion results from excessive exposure of tooth surfaces to dietary acids. It is defined as the irreversible loss of tooth structure due to chemical dissolution by acids or chelators that are not of bacterial origin.12 Although dental erosion has been recognized as a problem for decades, it is currently of great interest to the dental profession.
Recent public health surveys indicate that dental erosion is prevalent in most developed nations and often begins in early childhood.13 The causative acids come directly from the diet in the form of acidic-containing foods and drinks such as carbonated beverages, juices, energy and sports drinks, wine, salad dressings, etc. Single exposure to acid-containing products is not an issue. However, excessive intake of acid-containing products over time is a growing concern and the consumption of acid-containing beverages has increased by a staggering 500% over the past several decades.14 So while dental erosion was previously a relatively minor issue, it now has the potential to be a major factor affecting the retention of natural teeth into one’s senior years.
Dental erosion occurs as a result of tooth surfaces being repeatedly challenged with high concentrations of acid for relatively short periods of time. When exposed to excessive acid, the natural protective layer of pellicle, a protein film that covers and protects exposed tooth surfaces, is unable to withstand the level of challenge to which the teeth are subjected. In caries, teeth are exposed to low levels of acid for extended periods of time. Under this scenario, the pellicle is capable of protecting the tooth surface itself against dissolution, although acids are able to penetrate into subsurface regions of the tooth, eventually resulting in cavity formation.
In dental erosion, teeth are exposed to high concentrations of acid for relatively short periods of time; the protective pellicle is unable to protect against this level of challenge and the teeth begin to soften. Abrasive actions in the mouth such as brushing and eating are capable of removing this softened tooth mineral—even the tongue has the potential to remove erosively softened enamel due to continual friction with tooth surfaces. These surface related factors limit the potential for the softened tooth mineral to be remineralized, as occurs during the cavity-forming process. In the case of cavities, they begin to develop below the tooth surface, retaining structural integrity that can be reversed under the right conditions. However, in the erosion process, structural integrity is lost. For this reason, dental erosion is generally considered to be irreversible.12
Technical Approaches
Due to the irreversible nature of dental erosion, prevention rather than repair is the preferred technical approach15 and the prevention of dental erosion has taken a few different paths including additives to acid-containing products and the delivery of protective agents directly to tooth surfaces.
Calcium additives: Calcium additives, such as calcium citrate malate, calcium lactate and calcium fumarate, to acid-containing products have been shown to reduce the erosive potential of the aforementioned beverages.16–18 However, the inclusion of these additives can present formulation challenges due to the impact of the additives on product taste. In addition, while these compounds reduce the erosive potential at the point of insult, i.e., during use of an acid-containing product, they have not been demonstrated to remain on teeth for an extended time to provide a barrier of protection against subsequent acid attacks to the tooth surfaces.
Soluble phosphates: Soluble phosphates also have been used to reduce the erosive potential of beverages. Phosphates can attach to exposed tooth surfaces and create a barrier layer that helps to minimize erosive acid attack. Leading sources of phosphate include sodium hexametaphosphate and amorphous calcium phosphate. Both have been demonstrated to reduce the erosive potential of acid-containing beverages.19, 20
Fluoride: Finally, fluoride is wellknown for its ability to strengthen tooth mineral, enhancing its resistance to bacterial acids that cause cavities. While the extent of its benefits reported in early studies was disappointing,21, 22 later studies demonstrated that specific fluoride salts23 improved the benefits observed. Of the four fluoride salts commonly used in OTC toothpastes, stannous fluoride was shown to provide significant protection against dental erosion in both in vitro (see Figure 3) and in situ clinical trials (see Figure 4). Many studies also demonstrated the superior protection of stannous fluoride compared with other fluoride salts,15, 23-28 although all fluoride dentifrices are believed to provide some level of protection against erosion.22, 29
Discussion
As noted, it is now common for consumers to retain the majority of their natural teeth well into their senior years, thus it is critical to evaluate general tooth health in order to protect against other issues that can adversely impact longevity; tooth sensitivity and dental erosion are two such issues. Numerous technical approaches have been proposed for prevention and/or treatment of these conditions. Most of these are calcium-based and are incapable of withstanding strong dietary acid challenges. On the other hand, the strengthening benefits of fluoride have resulted in significant control over caries—although the question then becomes how to best protect teeth against new, emerging problems that go beyond the basics of cavity prevention.
The development of products capable of maintaining tooth health for life, regardless of challenges that arise, has been difficult. Delivery of an all-encompassing healthy tooth benefit in a single product formulation, such as in a toothpaste that is already in use by consumers, is an attractive proposition. Accomplishing this using one of the already proven and accepted fluoride salts is an even more attractive proposition.
Ensuring Formulation Success
Although consumers continually look for new oral care solutions in toothpastes, the delivery of clinically effective levels of fluoride is the most important benefit. In 1999, the US Center for Disease Control (CDC) issued a statement that water fluoridation is one of the 10 most important public health measures of the 20th century.30 Since water fluoridation is not available in many countries, toothpaste is considered to be one of the most important sources of fluoride, globally.31 As such, formulators must keep the need to maintain the anticaries efficacy of the product in mind, in addition to any new benefits. Importantly, the inclusion of new additives must not interfere with the overall aesthetics of the base formulation.32 Consumer researchers must work closely with formulators—who monitor the impact of additives on key technical measures such as rheology, color, flow, viscosity, pH and level of available active—to provide input regarding aesthetic parameters such as perceived color, shine, gloss, product stand-up, foam and flavor display during and after actual consumer use. In addition, dental researchers are crucial to the process, as they confirm the anticaries efficacy of the new product and ensure that any new benefit additives have not adversely impacted the release of the fluoride or its activity during brushing.
The measurement of soluble levels of actives in the formula alone does not provide sufficient perspective on the final clinical performance of toothpaste, although such measurements are generally the first level of concern. Between the 1950s and 1980s, full-scale clinical studies were the only broadly accepted means to ensure anticaries efficacy of fluoride-containing toothpastes. In 1995, the U.S. Food and Drug Administration issued a Final Caries Monograph, establishing guidelines for confirming the effectiveness of new formulations using a combination of laboratory and animal studies that closely mimicked caries clinical results.33 Leading dental researchers have also developed and recommended an alternate approach based solely on in vitro methods to confirm efficacy during the final stages of product development.34, 35 These methods make use of extracted human teeth and monitor the ability of oral care products to help prevent the initiation and progression of caries onto treated tooth surfaces under conditions that simulate human use of the products. The model results, correlated to both human and animal studies, provide important perspectives on the relative performance of new formulations. Similarly, in vitro models have been developed to simulate the in vivo processes leading to tooth sensitivity and erosion in order to evaluate the protective benefits of toothpastes.15, 36 These capabilities to simulate caries, sensitivity and dental erosion processes in the laboratory enable formulators to work closely with dental researchers to develop formulas with a high level of confidence prior to finalizing them for market.
Of particular concern to formulators is the fact that desensitization and precipitation technologies designed to address tooth sensitivity, and coatings designed to protect against dental erosion all have the potential to interfere with the anticaries efficacy of fluoride. Therefore, properly designed and well-controlled models must be used to ensure the stability and efficacy of products under simulated conditions of actual use. If desired, clinical studies are certainly possible, but well-designed in vitro modeling helps to ensure ultimate clinical success.
Alternative approaches to the use of fluoride for the prevention of caries have also been suggested, such as neem and mango,37 whose extracts are claimed to reduce bacteria that cause cavities; theobromine,38 an extract of cocoa beans that is believed to help strengthen enamel; and xyitol,39 a sugar alcohol used as a sweetening agent that does not promote caries due to the inability of bacteria to metabolize it—to name a few. One issue with these approaches is the difficulty in gaining their regulatory acceptance as new anticaries actives. The process for accepting new active agents is both expensive and time-consuming.
Rather than the development of alternatives to fluoride, the industry will most likely see ingredients developed to assist the accepted fluoride actives in working more efficiently. Enhancement in the bioavailability and retention of actives, such as has been accomplished with stabilized stannous fluoride, offers the potential to both enhance the base level of benefit and extend the benefits into new areas of emerging need.
Conclusions
Beyond cavity prevention, fluoride has been studied for its potential to protect teeth against other oral care problems such as tooth sensitivity and dental erosion, and of all the fluoride salts available, stannous fluoride is unique in its ability to fight cavities as well as reduce tooth sensitivity and protect teeth against dental erosion. Stannous fluoride deposits a protective barrier coating into dentinal tubules and onto exposed tooth surfaces, shielding these areas against acid attack. Although other formulation approaches have been developed to provide sensitivity or erosion protection benefits, they generally must be added to base fluoride formulations, and none have duplicated the ability of stannous fluoride to provide cavity, sensitivity and erosion protection in a single product.
References
- BG Bibby, Test of the effect of fluoride-containing dentifrices on dental caries, J Dent Res 24 297–303 (1945)
- KC Winkler, O Backer Dirks and J Van Amerogen, A reproducible method for caries evaluation. Test is a therapeutic experiment with a fluoridated dentifrice, Br Dent J 95 119–124 (1953)
- N Tinanoff, Progress regarding the use of stannous fluoride in clinical dentistry, J Clin Dent 6 (special issue) 37–40 (1995)
- DJ White, A “return” to stannous fluoride dentifrices, J Clin Dent 6 (spec iss) 29–36 (1995)
- ID Mandel, The new toothpastes, J Cal Dent Assoc 26(3) 186–190 (1998)
- DT Zero and A Lussi, Etiology of enamel erosion. Intrinsic and extrinsic factors, in Tooth Wear and Sensitivity, Martin Dunitz, London 121–139 (2000)
- DT Zero and A Lussi, Erosion. Chemical and biological factors important to the dental practitioner, Int Dent J 55 (suppl 1) 285–290 (2005)
- M Addy, Etiology and clinical implications of dentine hypersensitivity, Dent Clin North Am 34 503–515 (1990)
- Canadian Advisory Board on Dentine Hypersensitivity, Consensus-based recommendations for the diagnosis and management of dentine hypersensitivity, J Can Dent Assoc 69 221–226 (2003)
- S Kim, Hypersensitive teeth: Desensitization of pulpal sensory nerves, J Endodod 12 482–485 (1986)
- JA Kanapka, Over-the-counter dentifrices in the treatment of tooth hypersensitivity. Review of clinical studies, Dent Clin North Am 34 545–560 (1990)
- C Ganss, Definition of erosion and links to tooth wear, in Dental Erosion—From Diagnosis to Therapy, Karger: Basel (2006) pp 9–16
- T Jaeggi and A Lussi, Prevalence, incidence and distribution of erosion, in Dental Erosion— From Diagnosis to Therapy, Karger: Basel (2006) pp 44–65
- American Dental Association: Joint report of the American Dental Association on access, prevention and interprofessional relations and Council on Scientific Affairs to the house of delegates: Response to resolution 73H-2000 (Oct 2001)
- RV Faller, SL Eversole and GE Tzeghai, Enamel protection: A comparison of marketed dentifrice performance against dental erosion, Am J Dent 24 205–210 (2011)
- RE Davis, TA Marshall, F Qian, JJ Warren and JS Wefel, In vitro protection against dental erosion afforded by commercially available, calcium-fortified 100 percent juices, J Am Dent Assoc 138(12) 1593–1598 (2007)
- T Jensdottir, A Bardow and P Holbrook, Properties and modification of soft drinks in relation to their erosive potential in vitro, J Dent 33(7) 569–75 (2005)
- JA Hughes, NX West, DM Parker, RG Newcombe and M Addy, Development and evaluation of a low erosive blackcurrant juice drink. 3. Final drink and concentrate, formulae comparisons in situ and overview of the concept, J Dent 27(5) 345–350 (1999)
- A Lussi, Dental erosion—Novel remineralizing agents in prevention or repair, Adv Dent Res 21(1) 13–16 (2009)
- C Piekarz, S Ranjitkar, D Hunt and J McIntyre, An in vitro assessment of the role of tooth mousse in preventing wine erosion, Aust Dent J 53 22–25 (2008)
- ML Larsen and A Richards, Fluoride is unable to reduce dental erosion from soft drinks, Caries Research 36 75–79 (2002)
- JA Hughes, NX West and M Addy, The protective effect of fluoride treatments against enamel erosion in vitro, J of Oral Rehabilitation 31 357–363 (2004)
- AC Magalhães, A Wiegand, D Rio, MAR Buzalaf and A Lussi, Fluoride in dental erosion, in Fluoride and the Oral Environment, Karger, Basel (2011) 158–170
- T Willumsen, B Ogaard, BF Hansen and G Rolla, Effects from pretreatment of stannous fluoride versus sodium fluoride on enamel exposed to 0.1 M or 0.01 M hydrochloric acid, Acta Odontol Scand 62 278–281 (2004)
- SM Hooper, RG Newcombe, R Faller, S Eversole, M Addy and NX West, The protective effects of toothpaste against erosion by orange juice: Studies in situ and in vitro, J Dent 35 6 476–481 (2007)
- LH Hove, B Holme, A Young and AB Tveit, The protective effect of TiF(4), SnF(2) and NaF against erosion-like lesions in situ, Caries Res 42 68–72 (2008)
- N Schlueter, M Hardt, A Lussi, F Engelmann, J Klimek and C Ganss, Tin-containing fluoride solutions as anti-erosive agents in enamel; An in vitro tin-uptake, tissue loss and scanning electron micrograph study, Oral J Eur Sci 117 4 427–434 (2009)
- MCDNJM Huysmans, DHJ Jager, JL Ruben, DEMF Unk, CPAH Klijn and AM Vieira, Reduction of erosive wear in situ by stannous fluoride-containing toothpaste, Caries Res 45 518–523 (2011)
- C Ganss, J Klimek, U Schaffer and T Spall, Effectiveness of two fluoridation measures on erosion progression in human enamel and dentine in vitro, Caries Research 35 325–330 (2001)
- Ten Great Public Health Achievements—United States, 1900–1999, CDC Morbidity and Mortality Weekly Report 48 (12) 241–243 (Apr 2, 1999)
- D Bratthall, G Hänsel Petersson and H Sundberg, Reasons for the caries decline: What do the experts believe? Eur J Oral Sci 104 416–22 (1996)
- CS Newby, JL Rowland, RJ Lynch, DJ Bradshaw, D Whitworth and ML Bosma, Benefits of a silica-based fluoride toothpaste containing o-cymen-5-ol, zinc chloride and sodium fluoride, Int Dent J 61 3 74–78 (2011)
- U.S. Food and Drug Administration, Final monograph on anticaries drug products for over-the-counter human use, Fed Reg 60 52474–52510 (1995)
- JDB Featherstone, GK Stookey, MA Kaminski and RV Faller, Recommendation for a nonanimal alternative to rat caries testing, Am J Dent 24 289–294 (2011)
- GK Stookey et al, The Featherstone laboratory pH cycling model: A prospective, multi-site validation exercise, Am J Dent 24 322–28 (2011)
- DJ White et al, Stannous fluoride/sodium hexametaphosphate dentifrice increases resistance to tubule exposure in vitro, J Clin Dent 18 55–59 (2007)
- GM Prashant, GN Chandu, KS Murulikrishna and MD Shafiulla, The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivavius, Streptococcus mitis and Streptococcus sanguis: An in vitro study, Indian J Dent Res 18(4) 148–51 (2007)
- V DeMartini, PN Mason and GC Zuliani, Theobromine as a potential cariostatic agent, Friuli Med 24 525–529 (1969)
- S Mickenautsch and V Yengopal, Anticariogenic effect of xylitol versus fluoride—A quantitative systematic review of clinical trials, Int Dent J 62(1) 6–20 (2012)