Editor’s note: This article is the second in a four-part series considering the effects of test variables on SPF results. Here, the authors assess substrate choice; the first, published in July 2013, considered changes in substrate surface temperature. The third will consider an automated approach to controlling several test variables and will appear in the October 2013 issue.
Sun protection is currently a topic of concern due to the correlation between UV radiation and the incidence of skin cancer and skin aging. The determination of the level of protection a sunscreen product provides against ultraviolet (UV) radiation, expressed by SPF and UVA-PF, is historically based on in vivo methods and recent standards that have been published.1, 2 Further, for various reasons, in vitro methods are replacing in vivo methods; therefore, the reliability of in vitro evaluations is based solely on correlations with previous in vivo indexes. Test endpoints are determined in laboratory conditions and are made as realistic as possible, but reproducibility is the main factor in ensuring the reliability of the target for the in vitro method. To ensure inter laboratory reproducibility with the in vivo method, requirements include: a balanced volunteer panel, careful application, a controlled light source and a reproducible minimal erythema dose (MED) reading. In addition, one must consider the in vivo/in vitro correlation and repeatability—key parameters of which several previous studies have identified, including: quantity of product;3 spreading protocol; UV spectrum; temperature on the surface substrate;4 substrate properties such as surface energy5 or topographic parameters.6
Concerning this last parameter, different substrates have been proposed in recent years, including transparent tapea;7 quartz plates and skin equivalentsb, and polymethyl methacrylate (PMMA) plates. Initially, PMMA plates were produced using an uncontrolled sand blasting process, but this led to variability and poor reproducibility. However, when produced via a molded process, the resulting plates offer high reproducibility for both roughness and topographic parameters.6 For these reasons, Cosmetics Europe, formerly COLIPA, and ISO recommended plates with strictly controlled topographic parameters in their most recent methods and standards.8, 9
Regardless of the substrate, studies using numerous products have recently been conducted to evaluate the reliability of in vitro SPF evaluations, in terms of both repeatability and in vivo/in vitro correlation. The aim of this study was to determine just that, but it is only a first step toward improving SPF determination by means of an in vitro method and future harmonized method.
Materials and Methods
Substrate selection: Three types of PMMA plates with one face-roughness were purchased from suppliers: molded PMMA platesc measuring 47 x 47 x 1.5 mm—referred to here as MPP; molded skin-mimicking substrate platesd measuring 50 x 50 x 1 mm— referred to here as MSSP; and sandblasted PMMA platese measuring 50 x 50 x 3 mm—referred to here as SPP.
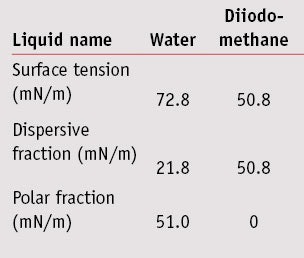
Surface free energy determination: The sessile drop method was used to determine each of the substrate’s surface energies and its polar and dispersive contributions. To characterize polar and dispersive components of the solid surface energy, the Wu method was used.7 For this method, at least two liquids with known polar and dispersive fractions are required. Here, the contact angle of waterf and diiodo-methaneg were measured by means of drop shape analysis systemh by placing a 5-µL drop of each on the substrate; the characteristics of these liquids are listed in the Table 1. For each surface energy measurement, nine contact angles—i.e., three plates and three spots per plate—were recorded for both water and diiodo-methane.
Topography assessment: Surface topography parameters were measured by means of a non-contact surface topographic analysisj on a surface area of 10 mm x 5 mm, in 15-µm intervals, with a speed of 100 steps/sec. This set-up was equipped with an XY plane spatial resolution of 0.1 µm and a confocal chromatic sensor with 3.39-nm vertical resolution (Z axis). Interpretation of the measurements was conducted by microtopography softwarek.
From these topography results, different parameters were selected according to both a previous study5 and typical standards for in vitro UVA-PF assessments:5, 6
Ra: the mean arithmetic deviation of the assessed profile from the average profile (µm);
Rv: the maximum depth of profile valleys within a sampling length (µm);
Rdq: the root-mean-square slope of the profile within a sampling length (expressed in degrees);
A1: the upper area, i.e., the area of the restovers of the peaks extending above an average profile ± kernel (µm²/mm);
Ssc: the arithmetic mean summit curvature of the surface, which indicates the mean form of peaks and valleys;
Vvv: the volume of void in the valleys, i.e., the volume of restovers of valleys extending below an average profile ± kernel (mL/m²); and
Sa: the mean arithmetic deviation of the assessed profile from the average surface (µm).
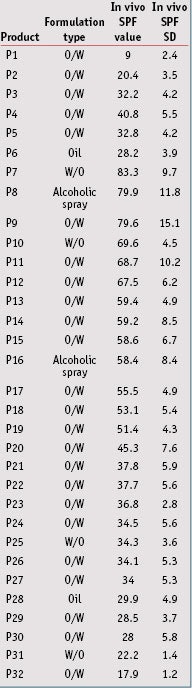
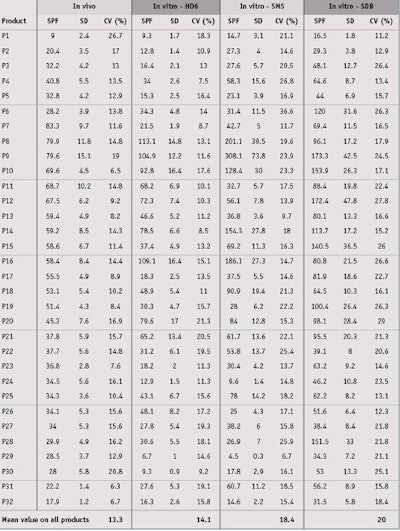
Sunscreen product selection: Thirty-two sunscreen products covering various formulations were chosen for this study. For all products, several measurements by different institutes had previously been taken, and in vivo SPF values ranged from 9 to 85 (see Table 2). These in vivo SPF values and standard deviations (SD) were determined according to the 2006 International SPF Test Method, as accepted by Cosmetics Europe; the Japan Cosmetic Industry Association (JCIA); the Personal Care Products Council (PCPC; formerly the Cosmetics, Toiletries and Fragrance Association or CTFA); and the South Africa CFTA (CTFA-SA).
Sunscreen application: Test sunscreen products were applied by a syringe across each plate surface. To ensure the correct application rate, the syringe was weighed before and after product application. The most common spreading protocol was chosen according to plate and substrate roughness; different common amounts of sunscreen product were applied depending on the type of plate in accordance with references; i.e., 28.7 ± 0.5 mg for MPP plates4 (equal to 1.3 mg/cm²), 50.0 ± 0.5 mg or MSSP plates8 (equal to 2.0 mg/cm²) and 32.5 ± 0.5 mg for SPP plates4 (equal to 1.2 mg/cm²).
Indeed, the quantity depended on the roughness because the aim was to provide the most efficient regularity of thickness. Immediately after weighing, the sunscreen product was spread over the entire surface using a defined sequence of circular and linear strokes according to substrate roughness. During spreading, application pressure was controlled by a pressure sensor, movements by video, and temperature by a device that controls and maintains the temperature at the surface of the plate—20°C in this study. After the product was spread, the sample was allowed to dry and settle for 15 min in the dark, again at 20°C. After drying, UV transmittance measurements were performed on the samples.
Transmittance measurements: According to the in vitro UVA-PF ISO 24443 procedure6 the transmittance analyzerm was validated and controlled by both specified standardsn and the linearity/additivity by calibrated reference standard PMMA platesp to which UV filters were incorporated. Measurements were taken at every nanometer in the 290-400 nm range. A PMMA plate covered with a film of white petrolatum, 15 mg applied, was used to obtain blank transmittance. Two different plates were used for each tested product, and nine UV transmission spectras were recorded for each plate at the different application locations.
In vitro SPF calculation: By means of sunscreen UV transmittance measurement, T(λ), the in vitro SPF could be calculated by combining the UV light attenuated by the sunscreen film, the erythema action spectrum, and a relevant solar emission spectrum according to Equation 1:
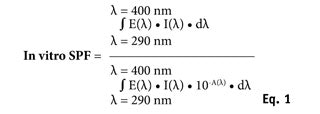
where E(λ) is the relative effectiveness of UVR in producing erythema in human skin according to the erythema action spectrum (CIE-1987) at wavelength λ; I(λ) is the spectral irradiance received from the UV source at wavelength λ, in this case calculating results from UV sources representing midday midsummer sunlight—UV spectra received at the earth’s surface in Zenithal conditions; A(λ) is the monochromatic absorbance of the sunscreen layer at wavelength λ, with A(λ) = –log [T(λ)]; and d(λ) is the wavelength step, equal to 1 nm in this study. Finally, the in vitro SPF reported in this study is the arithmetical mean of the 18 in vitro calculated SPFn values for each n location. The range of variation of in vitro SPF values is characterized through SD and coefficient of variation (CV).
In vitro/vivo SPF correlation: Two criteria were selected to determine the reliability of the in vitro SPF evaluations. First, the correlation between in vivo SPF values and in vitro SPF values through the well-known Pearson’s linear correlation coefficient, r. The closer that correlation coefficient r is to 1, the better the in vitro SPF measurements correlate with in vivo measurements. The second criteria was determining the root mean square error (RMSE). This criterion represents the distance between in vivo and in vitro values: the smaller the RMSE, the better accuracy. Finally, a linear regression using the well-known least squares (OLS) regression was performed, with zero as the fixed constant to show the tendency of in vitro SPF measurements to be higher or lower compared to ideal correlations with in vivo measurements.
Results and Discussion
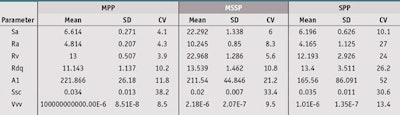
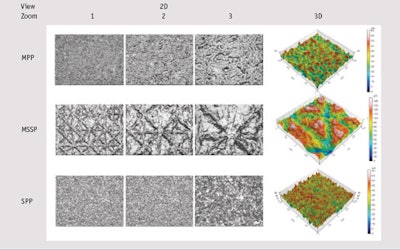
Substrate characterization: The surface topography of substrates was measured to compare topography parameters, the results of which are summarized in Table 3. Furthermore, surface images according to substrates are shown in Table 4.
From these results, it is important to note that topography reproducibility of the molded MPP were better than the MSSP and SPP. Indeed, variations of the different topography parameters of MSSP and SPP were very high with, for example, Ra values equal to 10.1 ± 8.3% and 4.2 µm ± 27.0% respectively. In contrast, the MPP Ra values equaled 4.8 ± 4.3%. Thus, the ranking of substrates based on the reproducibility of all topographic parameters, except for the Ssc parameter, was as follows: MPP > MSSP > SPP (more reproducible > less reproducible). Furthermore, it is interesting to note that the particular topography of the MSSP substrate is very different, and can lead to different topography values according to surface area measured.
The surface energies with dispersive and polar fractions of surface roughness also were calculated. Results are shown in Figure 1. Concerning surface roughness, surface energies were close between the MPP, SMS and SPP plates, with respectively 51.7 mN/m, 55.1 mN/m and 49.0 mN/m. Nevertheless, SPP were more hydrophobic, with a polar fraction of 2.9 mN/m compared with the MPP and SMS plates, with the polar fraction of about 10-12 mN/m. The absorbance spectrum assessment of substrates was performed using white petroleum; results are shown in Figure 2. Here, the SPP plates absorbed across the entire UVR 290-400 nm range, whereas molded plates MPP and MSSP did not absorb or absorbed very little.
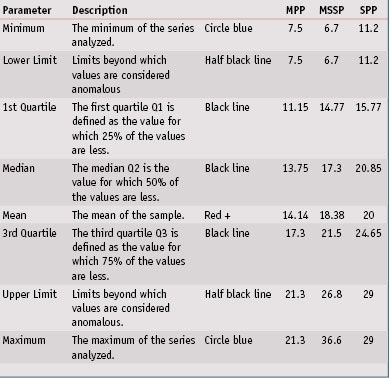
In vitro SPF evaluation: Next, the in vitro SPFs for all 32 products were evaluated on the three different substrates. Detailed results are shown in Table 5. Concerning repeatability of results, a box plot graphic, also referred to as a box and whisker diagram, shown in Figure 3, represents the in vitro SPF variations according to substrate. These univariate representations of quantitative data are a simple and complete representation of the minimum, first quarter, median, mean and third quarter displayed together with both limits—i.e., the ends of the “whiskers”—beyond which values are considered anomalous. The mean is displayed with a red plus sign (+), and a black line corresponds to the median.
By means of this graphic, it is clear that MPP provided less variability than other substrates. Indeed, the in vitro spreading method with the same operator allowed for the low average coefficient of variation of 14.1% for MPP substrates. Although MSSP substrates were also molded, a higher coefficient of variation of 18.4% was observed, possibly due to a high roughness value or slightly higher roughness variation compared to MPP substrates. Finally, the SPP plates exhibited the highest coefficient of variation of 20.0%; this is due to the way they are manufactured, which results in poor reproducibility intra and inter-plates. This had already been demonstrated in previous publications and confirmed is this paper with numerous sunscreen products. The detailed data and description of box plots parameters are shown in Table 6.
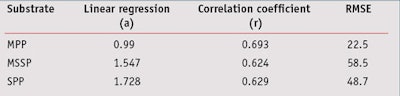
In vivo/In vitro SPF Correlation: From data presented in Table 5, the in vivo/in vitro SPF correlation was compared according to the three different substrates. Results shown in Figure 4, for the MPP substrate, led to a correlation coefficient of 0.693, which indicates the best correlation with in vivo SPF values compared to other substrates. Resulting linear regression follows the equation y = 0.990x; in general, in vitro SPF values were very slightly underestimated. Finally, the RMSE for the MPP substrate was lower than the other substrates, which indicates a better accuracy with in vivo values.
From results obtained with the MSSP or SPP substrates (see Figures 5 and 6), the correlation coefficients were close but a little lower than the MPP substrates, at respectively 0.624 and 0.629. The RMSE for these were worse than the MPP substrates, and linear regressions show that in vitro SPF values were overestimated in both cases. In the case for sandblasted plates where in vitro SPFs were systemically estimated as higher than the in vivo SPF, except on one occasion, the results were even dramatically worse. Correlation results with midday midsummer sunlight for the three different substrates are shown in Figure 7. Detailed results of linear regression, correlation coefficient and RMSE are summarized in Table 7.
Conclusion
Overall, from the various experiments performed in this study, it appears that molded MPP substrates were the most appropriate substrate for in vitro SPF assessments, compared with MSSP or SPP substrates. In fact, on a large set of products, it was demonstrated that MPP substrates allowed both better correlation and accuracy between in vivo SPF and in vitro SPF. Variability of results was also lower with MPP substrates. This had previously been demonstrated with recommendations by the COLIPA and ISO committees for such substrates.
Regarding other substrates, although in vivo/in vitro correlation was similar between MSSP and SPP, MSSP showed better results through repeatability, compared to SPP. Furthermore, SPP substrates always overestimated in vivo SPF values and could be dangerous in some cases (if the targeted value for in vivo SPF determination is based on in vitro SPF from an SPP value screening).
Finally, although repeatability is validating results in one’s laboratory, it seems logical to work on reproducibility improvements between different laboratories to assure a worldwide method for in vitro SPF assessment. Clearly, for a harmonized method, having correlation reproducibility between laboratories is also crucial.
References
1. US FDA, Department of Health and Human Services, Sunscreen drug products for over-the-counter human use, Final Rule 21 CFR parts 201 and 310, Federal Register 76 (117) (Jun 17, 2011)
2. ISO 24444: 2010, Cosmetics—Sun protection test method—In vivo determination of the sun protection factor (SPF)
3. BL Diffey and J Robson, A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum, J Soc Cosmet Chem 40 127–133 (1989)
4. M Pissavini, S Marguerie, A Dehais, L Ferrero and L Zastrow, Characterizing roughness: A new substrate to measure SPF, Cosm & Toil 124(9) 56-64 (Sep 2009)
5. COLIPA. Method for in vitro determination of UVA protection. COLIPA Guidelines (2011)
6. ISO 24443. Cosmetics–Sun protection test method–Determination of sunscreen UVA photoprotection in vitro, ISO/FDIS 24443:2011(E)
7. S Wu, Polar and nonpolar interactions in adhesion, Journal of Adhesion 5 39-55 (1973)
8. Y Miura, T Hirao and M Hatao. Influence of application amount on sunscreen photodegradation in in vitro sun protection factor evaluation: proposal of a skin-mimicking substrate, Photochem Photobiol, 88(2) 475-482 (2012)