Dry skin concerns many individuals, conferring discomfort and even impacting the lives of those severely affected. However, in most cases it can be adequately managed with current moisturizing products.1
Glycerol is one humectant commonly employed due to its high hygroscopic and hyperosmotic properties. It is used in cosmetics as a moisturizer and in pharmaceutical formulations as an active compound.2 Being naturally present in the skin, glycerol was quickly identified for its role in skin hydration, similar to natural moisturizing factors (NMF). A byproduct of triglyceride lipolysis, it is transported from the dermis through keratinocytes by a transmembrane water/glycerol transporting protein, aquaporin 3.3, 4
Glycerol also protects skin against irritant dermatitis and accelerates the recovery of irritated skin.5, 6 One in vivo study by Flurh et al.7 assumes that the stimulus for barrier repair occurs through water flux due to the hygroscopic properties of glycerol. Likewise, most studies about skin barrier recovery are performed with relatively high concentrations of glycerol, e.g., from 5% to 50%, often dispersed in an emulsion.
The question arises as to whether glycerol acts solely through its hygroscopic properties or implies more specific mechanisms. Indeed, diglycerol and triglycerol, with higher humectant activity, have demonstrated low improvements in skin dryness, compared with glycerol.8 Also, one in vivo attempt has been made to characterize the effects of low glycerol doses, e.g. from 1% up to 10%, on water transport through the skin after acute chemical irritation by sodium lauryl sulfate (SLS).9 The present article therefore summarizes and clarifies the benefit of glycerol on irritated skin.
Inducing and Assessing Irritation
Seven skin areas on the volar forearm of four female volunteers were delimited and treated for three hours with 200 µL of a 10% sodium lauryl sulfate (SLS) aqueous solution under occlusion with plastic closed chambers. One site was treated with 200 µL deionized water as a control site. Irritated skin areas presented as having disrupted barrier function and reduced hydration, as determined by biophysical measurements of transepidermal water loss (TEWL) and hydration.10
Following SLS treatment, TEWL increased 3.6 times, compared with basal values. However, this increase in TEWL value was partly due to occlusion, as the control site presented a 2.9-time increase compared to the basal value (see Table 1). Hydration, determined by capacitance measurements, decreased after SLS treatment under occlusion by approximately 40%.
Occlusion did not affect skin hydration, as values were similar to basal values. However, SLS itself did affect skin hydration. SLS is an anionic surfactant that induces, with high variability amongst individuals, intercellular inflammatory edema in the epidermis, leading to hyper-hydration and increased TEWL followed by dryness and scaling. Treating skin with SLS is thought to interfere with the lipid bilayer organization in the stratum corneum,11 and to modify the α-helical keratin protein conformations implicated in skin hydration.12-14 As a result, SLS increases hydrophilic but not lipophilic compound absorption;15 but this can be prevented through the addition of glycerol.16
Effects of Glycerol
Following acute chemical irritation, skin areas were gently dried with a cotton swab and exposed to increasing concentrations of aqueous glycerol solutions—i.e., 0%, 1%, 2%, 3%, 5% and 10% v/v, under occlusion for three hours. The control site was again exposed to deionized water under occlusion. After exposure, chambers were removed and the treated zones were gently dried with cotton swabs.
The increasing amounts of glycerol did not restore skin barrier function vs. the basal value. SLS induced irritancy was sustained and TEWL values continued to increase after removal of the chemical. However, the application of glycerol solutions above 3% v/v limited the TEWL values close to post-acute chemical irritation, preventing sustained action. And although the skin barrier function remained affected by SLS treatment after glycerol application, skin hydration was improved and even restored to initial values with 2% glycerol solution. This improvement in skin hydration increased with glycerol concentration, reaching a plateau above 2% . Thus, glycerol prevented the sustained action of SLS treatment and restored skin hydration with a plateau phase of action.
Conclusions
Taken together, these observations suggest that glycerol works by a mechanism based on its hygroscopic properties, which increase the water-holding capacity (WHC) of the impaired stratum corneum. WHC reflects and equilibrium between bound and free water, hydration (H) and TEWL values, respectively. Thus, the variation of WHC (ΔWHC) induced by glycerol treatment is determined from:
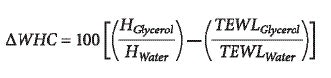
Variations of WHC as a function of glycerol concentrations applied on acute chemical irritation sites are depicted in Figure 1. It appears that glycerol greatly improved the WHC of the skin from concentrations as low as 1% v/v, whereas above 3% v/v, a plateau occurred, suggesting that the skin is saturated with bound water. From these observations, it appears that glycerol does not fully counterbalance the consequences of SLS interaction with skin, and mostly compensates the reduced WHC through its hygroscopic properties. However, improving cutaneous water content would help the physiological repair of the impaired barrier, notably through the activation of stratum corneum proteases responsible for the regulation of corneocyte desquamation.
To conclude, glycerol is efficient in preventing and adding recovery from chemical irritation induced by SLS. Its efficacy to restore skin hydration is significant at low doses, thus compliant with patients’ requirements and eligible for chronic treatment of constitutive skin dehydration, e.g., ichthyosis. and casual dry skin.
References
- M Lodén and HI Maibach, Treatment of Dry Skin Syndrome—The Art and Science of Moisturizers, Springer-Verlag, Berlin and Heidelberg (2012)
- L Roussel, N Atrux-Tallau and F Pirot, Glycerol as a skin barrier influencing humectant, in: Treatment of Dry Skin Syndrome, M Lodén and HI Maibach, eds, Springer-Verlag, Berlin and Heidelberg pp 473-80 (2012)
- M Hara-Chikuma and AS Verkman, Roles of aquaporin-3 in the epidermis, J Invest Dermatol 128(9) 2145-51 (2008)
- R Sougrat et al, Functional expression of AQP3 in human skin epidermis and reconstructed epidermis, J Invest Dermatol 118(4) 678-85 (2002)
- F Andersen et al, Anti-irritants I: Dose–response in acute irritation, Contact Derm 55(3) 148-54 (2006)
- F Andersen et al, Anti-irritants II: Efficacy against cumulative irritation, Contact Derm 55(3) 155-9 (2006)
- JW Fluhr et al, Glycerol accelerates recovery of barrier function in vivo, Acta Derm Venereol 79(6) 418-21 (1999)
- JW Fluhr, R Darlenski and C Surber, Glycerol and the skin: Holistic approach to its origin and functions, Br J Dermatol 159(1) 23-34 (2008)
- N Atrux-Tallau et al, Effects of glycerol on human skin damaged by acute sodium lauryl sulfate treatment, Arch Dermatol Res 302(6) 435-41 (2010)
- N Atrux-Tallau, Effects of physical and chemical treatments upon biophysical properties and micro-relief of human skin, Arch Dermatol Res 300(5) 243-51 (2008)
- M Boncheva, F Damien and V Normand, Molecular organization of the lipid matrix in intact stratum corneum using ATR-FTIR spectroscopy, Biochim Biophys Acta 1778(5) 1344-55 (2008)
- SM Patil, P Singh and HI Maibach, Cumulative irritancy in man to sodium lauryl sulfate: The overlap phenomenon, Intl J Pharmaceutics 110(2) 147-54 (1994)
- KP Wilhelm, AB Cua, HH Wolff and HI Maibach, Surfactant-induced stratum corneum hydration in vivo: Prediction of the irritation potential of anionic surfactants, J Invest Dermatol 101(3) 310-5 (1993)
- S Yadav et al, Comparative thermodynamic and spectroscopic properties of water interaction with human stratum corneum, Skin Res Technol 15(2) 172-9 (2009)
- A Chiang, E Tudela and HI Maibach, Percutaneous absorption in diseased skin: An overview, J Appl Toxicol 32(8) 537-63 (2012)
- S Ghosh, D Kim, P So and D Blankschtein, Visualization and quantification of skin barrier perturbation induced by surfactant-humectant systems using two-photon fluorescence microscopy, J Cosmet Sci 59(4) 263-89 (2008)