Manufacturers of topical products perform rigorous testing to assure that their products are safe for consumers. Of particular interest is determining whether products will irritate the skin of the approximately 50% of consumers surveyed who consider themselves to have “sensitive” skin.1-4
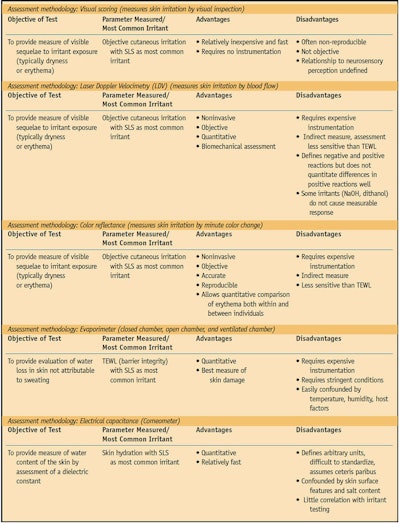
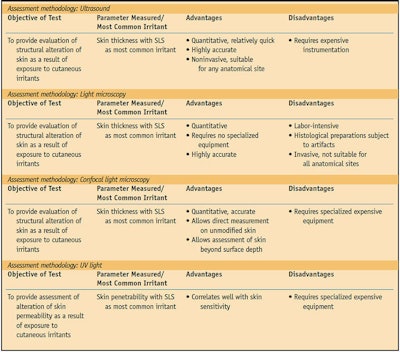
Several approaches have been used to study the irritation potential of cosmetics, soaps and other toiletries. Various chemical and mechanical irritants have been tested, as have several methods of assessing the response to irritants. The three primary testing methods include sensory reactivity tests, irritant reactivity tests and dermal function tests (see Table 1, Table 2 and Table 3).
Sensory Reactivity Tests
The objective of sensory reactivity testing is to measure an individual’s sensory response to burning, itching or other irritants in the absence of visible evidence of irritation such as erythema. This type of test allows the researcher to assess the individual’s subjective response to the irritant.
The sting test has been the most commonly used sensory reactivity test.5 Typically, lactic acid is applied to the skin, although other substances have been used such as capsaicin, ethanol, menthol,6 and sorbic and benzoic acid.7 Sometimes researchers will tape-strip the site of the skin test before applying the irritant to remove the stratum corneum (SC) layer (nonviable epidermis).
Various body sites can be used for testing, but the nasolabial fold commonly is used because this crease, running from the nose to the corners of the mouth, is highly sensitive. It has a permeable SC, a high density of apocrine glands and hair follicles, and is richly innervated.8, 9 Researchers assess the participants’ sensory responses to the irritant using a labeled-magnitude scale.10
The advantages of the sting test are that it is simple and relatively inexpensive to perform. However, the sting test response using lactic acid is not predictive of response using other irritants.9
Irritant Reactivity Tests
Irritant reactivity tests measure visible signs of irritation, such as erythema and dryness. The most commonly used irritant is sodium lauryl sulfate (SLS), an anionic emulsifier found in many cosmetics and other toiletries. Other irritants studied include dimethyl sulfoxide, benzoic acid and trans-cinnamic acid;6 lactic acid, octanoic acid and decanol;11 sodium dodecyl sulfate;6 and vasodilators.12 Physiological saline is often used as a “control” patch, although even this fluid is capable of producing skin irritation when applied under occlusive conditions.13
Irritant reactivity testing with SLS is conducted commonly by using several patch testing scenarios such as occluded14 and open application testing14, 15 and soak or wash tests,14 as well as repeat-insult patch testing.13, 15, 16 Researchers also use “exaggerated exposure” times and further exaggerate the effects of the irritant by adding a frictional component to the test method.10 Mechanical irritation testing methods are employed to evaluate the irritative effects of facial tissues17 and menstrual pad surface coverings.18
Typically, researchers visually grade irritant response as the amount of erythema and edema according to an industry accepted scale.8, 19 Erythema can also be measured by cutaneous blood flow,20, 21 plethysmography,14 color reflectance7 and laser Doppler velocimetry.21
Dermal Function Tests
Dermal function tests evaluate the structural alteration or physiological changes resulting from exposure to cutaneous irritants applied to sensitive skin. These tests most commonly involve evaluating transepidermal water loss (TEWL)22 as an indicator of the amount of skin damage,23 usually with SLS as the applied irritant. TEWL is more objective and specific than a visual scoring of irritation and is best correlated with SLS exposure.14 Ultrasound of the testing area after TEWL measurement to determine skin thickness shows good correlation with the results obtained by TEWL measurement.14
TEWL is affected by factors such as cutaneous blood flow as well as eccrine and apocrine gland density.22 The environment of the testing location must be controlled for temperature and humidity in order to obtain accurate results.10
Comment
In order to permit sensitive skin participants of dermatological studies to distinguish products correctly and consistently, it is important to develop effective, accurate and reproducible test methods that are minimally invasive. Current testing methods do not meet these goals. Newer testing methods under development will utilize technology to visually score evidence of irritation via polarized light and assess cytokine levels to determine clinical tissue damage.10
Editor’s note: The concluding article in this three-part series will discuss the relationship between irritant stimulation and sensory response in Sensitive Skin Syndrome. The current article is the continuation of a discussion on sensory response and classification.24 A more complete analysis of Sensitive Skin Syndrome is found in Reference 25.
Acknowledgments: The authors acknowledge the kind permission from Blackwell Publishing allowing this article to republish parts of the following original publication: MA Farage, A Katsarou and HI Maibach, Sensory, clinical and physiological factors in sensitive skin: A review, Contact Derm 55(1) 1–14 (2006).
References
1. R Jourdain, O de Lacharriere, P Bastien and HI Maibach, Ethnic variations in self-perceived sensitive skin: epidemiological survey, Contact Derm 45 162–169 (2002)
2. CM Willis, S Shaw, O de Lacharriere, M Baverel, L Reiche, R Jourdain, P Bastien and JD Wilkinson, Sensitive skin: an epidemiological study, Br J Dermatol 145 258–263 (2001)
3. MA Farage, How do perceptions of sensitive skin differ at different anatomical sites? An epidemiological study, Clinical Experimental Dermatology (In Press 2008)
4. MA Farage, Perceptions of sensitive skin: Changes in perceived severity and associations with environmental causes, Contact Derm (In Press 2008)
5. P Frosch and AM Kligman, Method for appraising the sting capacity of topically applied substances, J Soc Cosmetic Chem 28 197–209 (1977)
6. M Marriott, J Holmes, L Peters, K Cooper, M Rowson and DA Basketter, The complex problem of sensitive skin, Contact Derm 53 93–99 (2005)
7. S Seidenari, M Francomano and L Mantovani, Baseline biophysical parameters in subjects with sensitive skin, Contact Derm 38 311–315 (1998)
8. J Coverly, L Peters, E Whittle and DA Basketter, Susceptibility to skin stinging, non-immunologic contact urticaria and acute skin irritation; is there a relationship? Contact Derm 38 90–95 (1998)
9. DA Basketter and HA Griffiths, A study of the relationship between susceptibility to skin stinging and skin irritation, Contact Derm 29 185–188 (1993)
10. MA Farage, MV Santana and E Henley, Correlating sensory effects with irritation, Cut Oc Toxicol 24 45–52 (2005)
11. MK Robinson, Racial differences in acute and cumulative skin irritation responses between Caucasian and Asian populations, Contact Derm 42 134–143 (2000)
12. CJ Gean, E Tur, HI Maibach and RH Guy, Cutaneous responses to topical methyl nicotinate in Black, oriental, and Caucasian subjects, Arch Dermatol Res 281 95–98 (1989)
13. MA Farage, A Stadler, P Elsner, G Creatsas and HI Maibach, New surface covering for feminine hygiene pads: dermatological testing, Cut Oc Tox 24 137–146 (2005)
14. CH Lee and HI Maibach, The sodium lauryl sulfate model: an overview, Contact Derm 33 1–7 (1995)
15. N Muizzuddin, KD Marenus and DH Maes, Factors defining sensitive skin and its treatment, Am J Contact Derm 9 170–175 (1998)
16. MA Farage and HI Maibach, The vulvar epithelium differs from the skin: implications for cutaneous testing to address topical vulvar exposures, Contact Derm 51 201–209 (2004)
17. MA Farage, Assessing the skin irritation potential of facial tissues, Cut Oc Tox 24 125–135 (2005)
18. MA Farage and A Stadler, Risk factors for recurrent vulvovaginal candidiasis, Am J Obstet Gynecol 192 981–982, author reply 982–983 (2005)
19. Bioengineering of the Skin: Methods and Instrumentation, E Berardesca, P Elsner, KP Wilhelm and HI Maibach, eds, New York: CRC Press (1995)
20. H Loffler, H Dickel, O Kuss, TL Diepgen and I Effendy, Characteristics of self-estimated enhanced skin susceptibility, Acta Derm Venereol 81 343–346 (2001)
21. F Kompaore, JP Marty and C Dupont, In vivo evaluation of the stratum corneum barrier function in blacks, Caucasians and Asians with two noninvasive methods, Skin Pharmacol 6 200–207 (1993)
22. R Warren, A Bauer, C Greif, W Wigger- Alberti, MB Jones, MT Roddy, JL Seymour, MA Hansmann and P Elsner, Transepidermal water loss dynamics of human vulvar and thigh skin, Skin Pharmacol Physiol 18 139–143 (2005)
23. AB Cua, KP Wilhelm and HI Maibach, Cutaneous sodium lauryl sulphate irritation potential: age and regional variability, Br J Dermatol 123 607–613 (1990)
24. MA Farage and HI Maibach, Sensitive skin syndrome: Sensory response and classification, Cosmet Toil 123(7) 26–30 (2008)
25. Sensitive Skin Syndrome, E Berardesca, JW Fluhr and HI Maibach, eds, New York: Taylor and Francis (2006)