Cosmetics, shampoos and most personal care products are typically assessed for ocular irritation throughout the formulation process; however, they are required to be tested before the final product is released in order to provide proper warning labels. Otherwise, the manufacturer must indicate the product has not been tested. In fact, according to the US Food and Drug Administration (FDA), “A cosmetic is considered misbranded if its safety has not been substantiated and it does not bear the following conspicuous statement on the principal display panel (PDP): ‘Warning–The safety of this product has not been determined.’”1 Starting in 2013, most cosmetics and personal care products sold in Europe also will be required to include safety guidance on their labels.2
There are additional compelling reasons to test for ocular irritation. Primarily, products that irritate the eyes will obviously have limited market longevity and put brand reputations at risk. Since consumers can be impacted by even mild ocular irritation, the test used should be highly accurate in its assessment of mild and moderate irritants, as well as severe irritants. Currently it is up to formulators to determine which ocular irritation test is used.
Traditionally, ocular irritation testing has been conducted using rabbits. A test substance is applied to the conjunctival sack of rabbits under restraint for 4 hr, followed by a 14-day test period. After the first hour and on days 1, 2, 3, 4, 7 and 14, the immune response—i.e., redness, swelling and discharge, as well as tissue damage, are evaluated in three areas of the eye: the cornea, iris and conjuntiva.3 However, the restraint of the animals as well as the 14-day test duration have raised public concern over unnecessary animal suffering.4
One purpose of this test is to evaluate any opacity caused by the test substance in the cornea of the eye and to measure it. Any disruption to the pigmented iris surrounding the pupil, which is deeper within the eye than the cornea, is also measured, and the conjunctiva or tissue surrounding the eye is evaluated for redness, chemosis and discharge.3 Different designations are used to classify the various physiological responses—i.e., Draize, European Union (EU), Globally Harmonized System (GHS), US Environmental Protection Agency (EPA), Hazardous Materials Identification System (HMIS), Federal Hazardous Substances Act (FHSA), etc.; using the same animal data but with different weights given to the various responses measured, as well as different overall approaches to statistical analysis.5
Ocular irritation is distinguished from ocular corrosion based on reversibility. If, after 14 days, there is still an irritant response, the substance is classified as corrosive, but if the effect has reversed, the substance is classified as an irritant. The irritancy classification systems described above typically include substances that are corrosive within their most severe class—i.e., GHS and EPA “Cat I” or EPA “R41.” Ocular irritation is generally considered a less severe class, i.e., GHS “Cat 2A” and “Cat 2B”; EPA “Cat II” and “Cat III”; and EU “R36.” Nonirritant classes are listed as GHS “NC”, EPA “Cat IV” and EU “NC."6
Ocular Irritation Tests
As previously mentioned, there is broad-based objection and aggressive lobbying against cosmetic and personal care product testing using animals. The “not tested on animals” label appeals to a large segment of consumers. In addition, US regulators discourage animal testing for ocular irritation, and Europe will ban ocular testing for most consumer product labeling. Interestingly, at the same time that animal testing will be banned in 2013, Europe also will mandate safety guidance labeling;7 the United States is expected to follow suit.
There are a limited number of ocular irritation tests that do not require the use of animals. These tests include cell culture-based tests, egg-based tests, and biochemical tests based on damage to specific molecules and structures found within the eye. Additional tests are also available, including those based on excised animal eyes, slugs, microspheres and other organisms, but are not discussed here.
Cell culture-based tests typically involve applying the test substance to a di fferentiated human tissue grown in a petri dish to determine its toxicity based on the degree of cells killed by the substance a fter a fixed time.6 Tests based on fertilized eggs, or the Hen’s Egg Test Chorioallantoic Membrane (HET-CAM), measure changes to the vessels that extend from the developing yolk to the air cell within the egg; this primitive respiratory tissue or chorioallantoic membrane is the CAM. These vessels, present early in development before the egg is considered an animal, can be used to determine the irritancy and corrosivity of a test substance. It has been suggested that the vessels in the CAM are similar to those in the conjunctiva.6 When the test is conducted, the substance is applied to the CAM and changes to the vessels—typically lysis, coagulation and hemorrhage, are measured and used to predict ocular irritancy and corrosivity. The HET-CAM test is used in the United States and Europe for the in vitro prediction of ocular corrosivity; however, this assay has not been accepted as a stand-alone assay.6 Other options for testing ocular irritation without the use of animals are biochemistry tests, such as the one described here. These tests measure specific variables associated with the irritation score.
Synthetic Tissue-based Assay
A new synthetic tissue-based assay for ocular irritation has been developeda in which the substance to be tested is applied to a semi-permeable membrane, i.e., the “ocular membrane,” designed to model the outermost layer of tissue in the cornea. When the sample moves through the synthetic ocular membrane, it contacts a macromolecular matrix consisting of one component that is similar in structure and organization to macromolecules within the cornea, and a second component that predicts damage to molecules related to the immune response and cell viability. The test is constructed such that irritants induce a change in optical density, as measured with a spectrophotometer.
The degree of change in optical density, at defined wavelengths, is predictive of the corneal opacity response measured in animals and humans, and the degree of macromolecule disruption associated with the immune response and cell viability. Th ese levels of opacity and damage are proportionate to the sample’s irritation potential. The final prediction is derived by comparing five measurements from the test substance, whether an entire formulation or individual chemical, with a set of standards having defined irritancy values. A defined algorithm uses quantitative analysis to provide a GHS and EPA prediction.
The combination of a test that predicts corneal opacity and damage to macromolecules and a test that predicts damage to the conjunctiva fits the top-down or bottom-up approaches to irritation assessment8 as suggested by the National Institute of Environmental Health Sciences (NIEHS) and European Center for the Validation of Alternative Methods (ECVAM).9 Further, it provides increased statistical power to the prediction.10 However, most formulators rule out ingredients known to be corrosive, so a test with accuracy for mild and moderate irritation may be most appropriate for cosmetics and personal care products. Exceptions to this include green and natural product formulations. Green products, which are oft en cleaning agents, are formulated to reduce packing commonly by concentrating the active agents. Concentration can result in an increased risk of irritancy and corrosive properties. In addition, natural products may have unique organic molecules with unknown potentials for toxicity. As with all ingredients, these can be active alone or in synergy with the other ingredients. Therefore, corrosivity as well as irritancy should be evaluated throughout formulation as well as before product release.
The new assay predicts whether a substance is an irritant or corrosive and can be used to screen a variety of consumer products including cosmetics and personal care products. It also has advantages of reduced animal use, rapid turnaround-as fast as 48 hr, lower costs, excellent predictive value, and high reproducibility. Aspects of this assay are in fact similar to a previous methodb that is no longer used in its original form.11-14 Besides predicting both corneal opacity and macromolecule damage to indicate immune response and cell viability, the assay is harmonized with modern ocular irritation classifications, and defines the applicabilitydomain and clear protocols. With these improvements, the accuracy of the method was found to be nearly 90%. This article reports the results of a validation study conducted using the biochemical ocular irritation assay.
Pre-screening and Application Domain
Prior to a full 24-well plate study, a defined set of objective tests are performed on the substances to be tested to confirm that they are within the assay's application domain since deviation from the application domain reduces the accuracy of the prediction. Specific physical and chemical measurements are a standard part of the pre-screening process and ensure that the test will be used only on appropriate test substances and that the appropriate protocol can be followed. In some cases, a substance is determined to be outside of the application domain and is therefore not considered as a candidate for the test method.
Biochemical Test Protocols
Based on the pre-screening results, one of three protocols is selected, with a fourth sub-protocol possibly being required. Surfactant samples are assayed using the Surfactant Assay (SA); samples that buffer in the highly alkaline or acidic range are assayed using the Alkaline/Acid Membrane Assay (AMA); and non-surfactant neutral samples are assayed using the Upright Membrane Assay (UMA). Specific details of each assay are provided here.
Upright Membrane Assay (UMA): 25 μL, 50 μL, 75 μL, 100 μL and 125 μL, or μg if solid, of the test substances are applied to an ocular membrane and incubated for 24 hr. The active agents in wells below the disks are assayed spectrophotometrically and the results are compared with a set of standards assayed at the same time as the sample. From this, an irritation prediction is calculated.
Surfactant Assay (SA): In this case, the test sample is solubilized and further diluted to five concentrations ranging from 1% to 50%. The sample is added to the test well and results are measured after 24 hr. Results are compared to a set of surfactant standards and an irritation prediction is calculated.
Alkaline/Acid Membrane Assay (AMA), either non-surfactant or surfactant: This protocol is similar to the UMA and SA protocol but used for substances with pH extremes. Specific standards and controls are used to control for pH-related assay interference and for quality control.
Intensely colored (IC) sub-protocol: When intensely colored samples may produce a background, this protocol is used. This sub-protocol uses spectrophotometry to identify wavelengths without interference from the colored sample, and the assay is conducted at wavelengths that do not interfere. Standards and quality controls are assayed at the same wavelength as the test substances. In some cases, the colored sample will absorb in all wavelengths and cannot be evaluated; it is therefore judged to be outside of the application domain.
Materials and Methods
Once a protocol is determined, to conduct the studies, five doses of the test substance are applied to separate ocular membranes. Each standardized, quality-controlled membrane covers the test well of a 24-well plate and within each well is an active agent. In its entirety, with all the samples, standards and quality controls, the test requires most of the 24 wells. After the test substance is applied, the plate is sealed and incubated for 24 hr. The plate is then removed and the active agent is analyzed with a spectrophotometer. Figure 1 shows an example of an irritant and nonirritant after the 24-hr incubation period. The irritant induced the opacity of the active agent similar to the corneal opacity measured in animals and humans. Assays were conducted using four test substances over the course of several weeks by adhering carefully to three specific assay requirements: the objective characterization of the substance to be tested, limiting testing to the defined application domains as identified by the pre-screening, and adherence to the standard operating procedures for the method. The measured values of the standards are then used to construct a curve to extrapolate an irritancy classification and score prediction for the substance under investigation.
Quality control checks are then conducted. Results are tested for false negatives; in some cases, substances that are strong denaturants can overwhelm the assay. In this event, the internal checks are able to identify false negatives and the agent is considered to be incompatible. The irritation score, based on standards and quality checks, is calculated and analyzed via a defined algorithm that determines assay and sample result acceptance based upon defined statistical parameters.
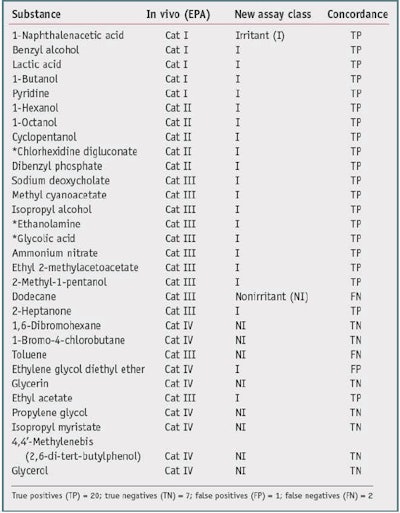
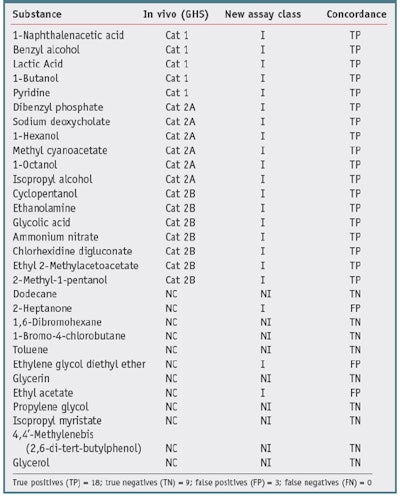
One goal of the new assay is to reduce animal testing, therefore no new animal testing was carried out for the validation trials. Also, due to the fact that most formulators do not share formulations or animal data, a high quality, publicly available source of animal test results was required to serve as the gold standard with which to compare test chemical results. One such source is the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) recommended reference substance list.10 This list contains consensus irritancy classifications for a number of chemicals.The researchers selected validation chemicals based on availability, cost, diversity of class, and diversity of irritancy classification. This list is provided in Table 1 and Table 2. The test chemicalsc used were all greater than 95% purity.
Results and Discussion
Thirty chemicals were selected based on the availability of reliable in vivo gold standard data, i.e., GHS and EPA. These chemicals were screened to assure they were compatible with the assay, the standard chemical and physical property evaluations were carried out, and the appropriate assay protocol was conducted. Results are presented as nonirritants versus the other chemicals. Table 1 and Table 2 show the results of the studies.
Table 1 shows a comparison with the U.S. National Toxicology Program's (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) EPA Consensus 1 GHS irritancy classifications, compared with a nonirritant and other chemicals. The new biochemical assay accurately classified 27 of the 30 test chemicals, resulting in an overall concordance (accuracy) of 90%. From this set, there was one false positive out of 10 negatives, resulting in a specificity of 90%, and there were two false negatives out of 20 positives, giving a sensitivity of 90%.
Table 2 shows the results compared with NICEATM GHS Consensus 1, 3. When compared with GHS consensus data, the new assay correctly predicted 27 out of 30 of the chemicals, resulting in a concordance (accuracy) of 90%. There were three false positives, such that nine of the 12 nonirritants were identified, resulting in a specificity of 75%. Finally, 18 of 18 irritants were correctly identified, resulting in a sensitivity, for this data set, of 100%.
It is interesting to note that just by comparing the GHS values with the EPA values of the nonirritants versus the other chemicals-in this case, for dodecane, 2-heptanone, toluene and ethyl acetate-the concordance is 26/30 or 80%. The two classification systems differ in the statistical analysis of the animal data and therefore are not harmonized. In general, the EPA classification system has a lower sensitivity limitation. The authors thus conclude that the new biochemical assay has a better specificity when compared with the EPA irritancy classification system, and a better sensitivity when compared with the GHS system classification system-for this data set. The overall accuracy was similar for both comparisons (90%).
Conclusions
The described synthetic tissue-based in vitro ocular irritation assay is a rapid way to evaluate irritation potential. The study presented here demonstrates that it is also a sensitive, specific and accurate way to determine ocular irritation potential. Such a test allows cosmetic and personal care product formulators to screen a broader range of chemicals and natural products prior to the final formulation. In addition, this type of test allows formulators to test products for irritation prior to product release, thereby reducing the risk to the public while at the same time reducing the suffering associated with animal testing.
References
All websites accessed May 29, 2012.
- www.fda.gov/Cosmetics/CosmeticLabelingLabelClaims/CosmeticLabelingManual/ucm126444.htm#clgk
- https://eur-lex.europa.eu/legislation_summaries
- http://iccvam.niehs.nih.gov/SuppDocs/FedDocs/EPA/EPA_870_2400.pdf
- https://www.peta.org/issues/animals-used-for-experimentation/animals-used-experimentation-factsheets/government-required-animal-testing-overview/?nowprocket=1
- http://iccvam.niehs.nih.gov/docs/ocutox_docs/InVitro-2010/AppJ-Analysis.pdf
- http://iccvam.niehs.nih.gov/methods/ocutox/ivocutox/ocu_brd_hetcam.htm
- https://eur-lex.europa.eu/legislation_summaries
- L Scotta et al, A proposed eye irritation testing strategy to reduce and replace in vivo studies using bottom-up and top-down approaches, Toxicol In vitro 24(1) 1-9 (Feb 2010)
- http://ecvam.jrc.it/publication/strategy.pdf
- http://iccvam.niehs.nih.gov/meetings/6thWC/posters/HattanSubstance.pdf
- RJ Soto and VC Gordon, An In vitro method for estimating ocular irritation, Toxicol In vitro 4(4-5) 332-335 (1990)
- Ibid Ref 15, pp 314-7
- VC Gordon, CP Kelly and HC Bergman, Evaluation of the Eytex system for use as a predictor of ocular irritancy: 1. Shampoo appli¬cations (12)1 35-47 (1993)
- D Decker and R Harper, Evaluation of the Eytex system for use as a predictor of occular irritancy: II. Conditioners and styling aids (12)4 371-380 (1993)





![A 2019 petition to the House of Commons stated, 'We, the undersigned residents of Canada, draw the attention of the House of Commons ... [that] animal testing is unnecessary to prove the safety of cosmetic products.'](https://img.cosmeticsandtoiletries.com/files/base/allured/all/image/2023/01/animal_testing_ban_canada_dreamstime_m_215632720.63d313232306d.png?auto=format%2Ccompress&fit=crop&h=191&q=70&rect=0%2C73%2C1800%2C1013&w=340)



