Film-forming polymers are used in diverse cosmetic formulations, such as for hairstyling, hair conditioning or sun care. Like other cosmetic ingredients, polymers end their lives in water; either in domestic wastewater or directly in environmental water. In order to address the rising awareness of the environmental fate of products, the cosmetic industry constantly seeks new solutions to reduce the environmental impact by careful ingredient selection. However, in applications with high performance requirements, such as hairstyling, the change toward more sustainable ingredients is especially challenging.
Furthermore, and in the particular case of polymers, there is a lack of clear and relevant information regarding their environmental fate. The majority of currently used synthetic film-forming polymers, such as acrylates or vinyl pyrrolidones (VP), do not biodegrade well and present the risk of persisting in the environment. This is attributed to the fact that such polymers, obtained by the radical polymerization of vinyl monomers, possess a backbone composed of carbon-carbon linkages that cannot be easily cleaved by enzymes.1
On the other hand, natural polymers are assumed by consumers to biodegrade better. However, just as for synthetic substances, the level and kinetics of biodegradation may strongly vary depending on the chemical structure of the polymer. Furthermore, natural polymers are often modified for the purposes of the application, which may significantly impair their biodegradability. This is sometimes the case with cellulose, which, when modified into carboxymethyl cellulose or hydroxyethyl cellulose, loses its ability to biodegrade with increasing degree of modification.2
It also is known that synthetic polymers with heteroatoms in their backbone, such as some polyesters or polyurethanes, can be easily degraded by microorganisms.3 Nevertheless, the biodegradation ability and kinetics will strongly depend on the specific molecular structure and accessibility of the cleavable sites by enzymes. It is therefore necessary to investigate the biodegradability of each particular substance.
Biodegradability
The biodegradability of materials can be determined according to various standardized methods, e.g., ASTM, ISO and OECD, depending on the environment—soft water, sea water, compost, etc.—and physical state of the sample. For polymers dissolved or dispersed in water, the OECD 301 and 302 methods are recognized standards.1 These test methods were initially developed for small organic molecules that typically biodegrade quickly, which explains the relatively short, approx. 28-day test duration; although high molecular weight polymers may take longer to degrade.
Materials and Methods
In the present work, tests were performed using the setup and sludge described in the OECD 301B guideline to assess aerobic readily biodegradability in domestic wastewater. This method is applicable to substances that are both soluble and poorly soluble in water.4
Materials: Film-forming polymers typically used for hairstyling were tested, including two polyester-based polyurethanes, polyurethane-48 and polyurethane-34; and a polyacrylate copolymer, octylacrylamide/acrylates/butylaminoethyl methacrylate copolymer. The acrylate copolymer was fully neutralized using sodium hydroxide prior to testing, in order to be fully solubilized without adding any carbon or nitrogen not bound to the backbone—which could otherwise influence the results. The other polymers were used without pretreatment. The two polyurethanes used were slightly anionic and contained sulfonic acid sodium salts in the polymer chain. Schematic molecular structures of the tested products are shown in Figure 1.
Test protocol: The test samples were placed in the bottle containing a polyvalent inoculum—i.e., the bacterial sludge from a sewage treatment plant that treats predominantly domestic sewage—and incubated at 21°C. During the test period, the solutions were stirred using a magnetic stirrer.
It is known that synthetic polymers with heteroatoms in their backbone, such as some polyesters or polyurethanes, can be easily degraded by microorganisms.
Degradation was followed by analyzing the CO2 released. The CO2 was collected using a Ba(OH)2 trap and titrated using a HCl solution and phenolphthalein as a color indicator, following the procedure described in the guideline. The amount of CO2 produced during the degradation of the test item was expressed as a percentage of the theoretical CO2 amount the test item could produce if all of its carbon atoms were turned into CO2.
Two control samples were run in parallel to validate the test measurements. To confirm adequate sludge activity, sodium benzoate, an easily biodegradable ingredient, was measured. Its successful biodegradation indicates normal activity of the sludge. The sodium benzoate control is compulsory to confirm the validity of the test.
The second control, for toxicity, is composed of the sodium benzoate together with the test item. This measurement indicates whether any side products are released during the degradation test that could be potentially toxic to microorganisms. The release of toxic side products is indicated by a lower level of CO2 release for the toxicity control sample in comparison with the sodium benzoate sample.
Biodegradation Process
The biodegradation of polymers proceeds through the enzymatic hydrolysis of hydrolyzable bonds, resulting in a decrease in molecular weight. This step can be accelerated by chemical hydrolysis or physical treatments such as heat, UV light, etc. The low molecular weight degradation products that are formed pass through the microorganism cell membrane and are further metabolized.1, 3 Based on this mechanism, shorter chains of a polydispersed polymer should degrade first.
As stated, the overall level of biodegradation of a polymer depends on the accessibility of cleavable bonds by enzymes, which can vary with the molecular structure. It is therefore possible that only the low molecular weight fraction of a polymer biodegrades and the biodegradation stops for polymer chains above a certain molecular weight threshold. It is also possible that degradation takes place at pendant groups containing heteroatoms or cleavable bonds, while the polymer backbone stays unmodified. However, these scenarios cannot be discriminated on the sole interpretation of CO2 evolution tests and require complex studies of degradation products. It is also worth noting that levels of 100% biodegradation are usually not reached, since some carbon is used by microorganisms to generate new biomass.
Biodegradation Results
The results obtained for the tested film formers are shown in Figure 2 and Figure 3. Figure 2 shows the degradation curves for polyurethane-48, the reference sample (sodium benzoate) and the toxicity control sample (sodium benzoate + polyurethane-35). The three experiments resulted in steady CO2 evolution curves, with no drop in CO2 emission during the test period. This suggests that neither the polymer nor its degradation products show toxic effects on microorganisms. Though not depicted here, toxicity was also not observed for the other polymers tested in this study.
The acrylate copolymer, although being fully soluble in water, showed no biodegradation during the designated test period. This result is in accordance with previous studies showing that only polyacrylates with a low molecular weight, i.e., below 7 monomer units (500-700 g/mol) in the case of sodium polyacrylate, can be partially metabolized by microorganisms. Meanwhile, the higher molecular weight fraction, which is the largest portion of the product, stays unaffected.5, 6
The two polyurethanes started to degrade after a few days of incubation. This period corresponds to the time required by microorganisms to adapt to a new substrate. Literature studies reported that PVP does not biodegrade in water. It was shown that PVP does not degrade in vivo and poorly adsorbs onto sludge, causing it to pass through wastewater treatments and accumulating in the environment.7
On the other hand, both polyurethanes reached approximately 50% biodegradation in 20 days. This level suggests all polymer chain lengths are affected by biodegradation, including longer ones, and indicates these polymers could be fully metabolized. The observed results on polyurethane biodegradation are in line with the literature results. In fact, the biodegradability potential of polyurethanes has attracted recent attention, including the search for and identification of several enzymes that are capable of degrading polyurethanes;9 and interest for biomedical applications, since polyurethanes show excellent biocompatibility.8, 9
Most currently used synthetic film-forming polymers, such as acrylates or vinyl pyrrolidones, do not biodegrade well and pose a risk of persisting in the environment.
Hairstyling Performance
In the case of hairstyling products, film-forming polymers constitute the main ingredient. Their function is to provide mechanical properties such as a manageability or hold, and to protect the hair from the influence of heat, humidity or water.
Indeed, consumers expect a styling product to maintain its hold under any humidity condition, even after short contact with water. Of course, the same requirements apply to biodegradable polymers; however, a high level of biodegradability is usually accompanied by high hydrophilicity and low hydrolytic stability, which in turn can affect the application performance.
Materials and Methods
Curl retention: To test the styling performance of the same four film formers on hair, curls were prepared by treating undamaged Caucasian strands with a 2% w/w film-former solution. The solutions were applied to wet hair at a rate of 1 g solution/1 g hair. The strands were wrapped around a curler, blown dry for 3 min at 75°C, and left to dry over night at RT under controlled 55% RH.
A first set of curls was used to measure the high humidity curl retention of the polymer films. Curls were placed in a humidity chamber for 5 hr at 21°C and 90% RH, and the percentage of curl retention over time was calculated as shown in Equation 1.
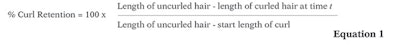
Curl retention results: Results of the hairstyling performance tests are depicted in Figure 4. Both polyurethanes and the acrylate copolymer achieved curl-retention levels above 60%, although the polyurethanes better retained the curl shape. This indicates less plasticization of the film by permeated water.
Water resistance: A second set of curls was used to test the water resistance of the polymer film. In this experiment, the curls were dipped in warm (38°C), soft water for 30 sec then removed and left to dry in a hanging state at RT and 55% RH.
Water resistance results: The photographs of the obtained hair strands are shown Figure 5. After contact with water, hair treated with PVP fully lost its shape, while hair treated with polyacrylate and the poylurethanes maintained a curl. This indicated better adhesion of the polymer film to the hair surface. Of all the curls, the one treated with polyurethane-48 showed the best shape retention.
Conclusions
In this study, polyurethane-48, polyurethane-34 and an acrylate copolymer, all typically used for hair care applications, were investigated for their biodegradability in water, and their water and humidity resistance when applied to hair. While results indicated polyacrylate was not biodegradable, polyurethane-based film formers showed excellent biodegradability in water. These results are in line with previous literature studies, which have already demonstrated the ability of well-designed synthetic polyurethanes to biodegrade. At the same time, polyurethanes showed the best hairstyling performance under humid or wet conditions, in terms of curl retention and water resistance.
References
- Eubeler, J. P., Bernhard, M., Zok, S., and Knepper, T. P. (2009). Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. Trends in Analytical Chem, 28, 1057-1072.
- VanGinkel, C. G., and Gayton, S. (1996). The biodegradability and nontoxicity of carboxymethyl cellulose (DS 0.7) and intermediates. Environmental Toxicol and Chem,15(3), 270-274.
- Lucas, N., Bienaime, C., Belloy, C., Queneudec, M., Sylvestre, F., and Nava-Saucedo, J. F. (2008). Polymer biodegradation: Mechanisms end estimation techniques–A review. Chemosphere, 73, 429-442.
- OECD 301: Ready biodegradability (2018, Jan. 1). OECD Guideline for Testing of Chemicals Adopted by the Council on 17th July, 1992. Retrieved from https://www.oecd-ilibrary.org/environment/test-no-301-ready-biodegradability_9789264070349-en
- Larson, R. J., and Bookland, E. A. (1997). Biodegradation of acrylic acid and oligomers by mixed microbial communities in activated sludge. J Environmental Polymer Degradation, 5, 41-48.
- The Soap and Detergent Association, (1996). Polyacrylates. Retrieved from https://www.aciscience.org/docs/Polycarboxylates.pdf
- Julinova, M., Kupec, J., Slavik, R., and Vaskova, M., (2013). Initiating biodegradation of polyvinylpyrrolidone in an aqueous environement: Technical note. Ecological Chemistry and Engineering, 20(1), 199-208.
- Loredo-Trevillo, G., Gutiérez-Sanchez, R., Rodriguez-Herrera, C. N., and Aguilar, J. (2012). Microbial enzymes involved in polyurethane biodegradation: A review. Polym Environ, 20, 258-265.
- Labow, R. S., Meek, E., and Santerre, J.P., (1999). The biodegradation of poly(urethane)s by the esterolytic activity of serine proteases and oxidative enzyme systems. Journal of Biomaterials Science, Polymer Edition, 10(7), 699-713.








!['[Sunscreen] developers will be able to innovate more efficiently while maintaining high standards of quality and safety for consumers.'](https://img.cosmeticsandtoiletries.com/files/base/allured/all/image/2024/06/woman_outside_using_sunscreen_on_face_ISO_test_standards_AdobeStock_783608310.66678a92029d9.png?auto=format%2Ccompress&fit=crop&h=191&q=70&rect=62%2C0%2C2135%2C1200&w=340)