New approaches to skin cleansing are gaining interest, and modern concepts for wellness respect the body’s and skin’s equilibrium, along with the environment1, 2—hence, the wide success of natural ingredients. This has led to the development of new detergent structures for improved benefits. To develop a new, wellness-inspired raw material, the authors began their search with vegetable oils, waxes and vegetal actives.
Choices of vegetable-derived, eco-friendly and mild surfactants are abundant, but, unfortunately, often do not match the level of functionality and exhibit the desirable sensory characteristics of synthetic ingredients. Further, many are incompatible with polymers, conditioning ingredients and traditional surfactants. They also have difficulty in reaching high viscosity values or, in some cases, even reaching a minimum viscosity. There are also too few options for transparent formulae. From a sensory standpoint, the main issues with vegetable-derived and mild surfactants are their foam quality, quantity and stability—key sensory factors for consumers. So, to guarantee a product’s shelf-life and preserve its sensory characteristics, the authors’ search was extended to other ingredient categories.
Beginning with olive and coconut oil fatty chains, sugars from fruits and amino acids, years of systematic trials culminated in a combination of specialized amino acid-fructoside surfactants: olivoyl/cocoyl fructoside (a non-ionic) and sodium olivoyl/cocoyl tetra amino acid (an anionic). This paper will describe the basis for this combinationa and detail tests conducted to verify the functional, sensorial and mildness characteristics.
Non-ionic/Anionic Blend
Non-ionic portion: The lipid structures of olivoyl/cocoyl fructosides are made up of the natural chain blends present in olive and coconut oils. Olive oil was selected for its high amount of mono-unsaturated fatty acids (75%) and because it is well-known for its compatibility with the human body.3, 4Moreover, its lipid moiety contributes emollient effects, normalizing and protecting skin.5 Coconut oil, also widely used for its emollient properties, has been found to moisturize atopically dermatitic skin while also removing Staphylococcus aureus. This effect is probably due to its lauric acid, which is known to kill bacteria and fungi.6
To achieve mildness while retaining high efficiency in terms of foaming capacity, compatibility and ease of thickening, the authors engineered hydrophilic heads by esterifying the fatty acid chains with the molecules of fructose (see Figure 1). Surfactants having non-ionic hydrophilic heads made from a sugar moiety are generally very mild, and can also reduce the harshness of traditional surfactants without ethoxylated impurities.
Anionic portion: The second ingredient in the combination, sodium olivoyl/cocoyl tetra amino acid, is an anionic molecule inspired by the concept of “interrupted” or “extended” soaps. These terms refer to a zone of intermediate polarity within the molecule, between the non-polar fatty alkyl moiety and the very polar anionic head. When these molecules come into contact with water, they create crypto-anionic hydration spheres in which the negative ionic charge is shielded by the layer or spherical zone of intermediate polarity, making the surfactant milder. This zone is created by linking specific amino acid or peptide chains to fatty acid chains.
It is well-known that amino-acid based ingredients are highly biodegradable, and N-acyl-amino acids, in particular, are mild. They also create an elastic, creamy foam. Further, they have been shown to retain moisture in hair.7 On the basis of these properties, studies were conducted using the alkaline salts of this amino-acid based surfactant,8-10 and four amino acids were finally selected and tested as possible chain extenders: valine (non-polar), alanine (non-polar), threonine and glycine (both polar). These four belong to the standard amino acid family; threonine and valine specifically are essential amino acids (see Figure 2 and Figure 3).
Physical Assessments
To evaluate the functional properties of the new olivoyl/cocoyl fructoside (OF) combination, a series of tests was carried out.
Foaming: To test foam capabilities, binary and ternary systems of the OF blend with other surfactants were evaluated, including: sodium laureth sulfate (SLES), disodium cocoamphodacetate (DCA), disodium laureth sulfosuccinate (DLS), coco-glucoside (CG) and PEG-7 glyceryl cocoate (PGC). A 100-mL sample of the standard 5% active test solution of surfactant or binary/ternary mixture was introduced to a 400-mL beaker. Acclimated to 25°C, the sample was then homogenized for 1 min at maximum speed and poured slowly into a 250-mL glass cylinder having a diameter of 35 mm. Foam height was measured after 1 hr (h0) and 5 hr (h1), and foam stability was calculated as a percentage of h1 to h0.
Thickening: The ability of the OF blend to thicken was tested using 14% active solutions of the blend (typical for shampoos) with thickeners commonly used in cleansing systems. These included NaCl, MgCl2 and CaCl2 salts at various concentrations; cocamide DEA at 2.0%; cocamidopropyl betaine at 3.0%; PEG-120 methyl glucose dioleate at 2.0%; and PEG-90 glyceryl isostearate laureth-2 at 5.2% or acrylates/steareth-20 methacrylate copolymer at 0.4%. These percentages were chosen based on the minimum amount required to achieve a viscosity value higher than 1,000 mPa•s. The ingredient PEG-120 methyl glucose dioleate was selected to test the same properties in comparison with the surfactants used in the foaming tests, and in binary and ternary systems. Viscosity values were measured with a viscometer.
Compatibility: Additional tests were carried out in order to verify the OF blend behavior at different pH levels and with additional ingredients that are often used in cleansing products—such as conditioners, polymers, preservatives, etc.
Mildness Assessments
While the chemical characteristics of the two surfactants and ratio of their combination in the OF blend were carefully optimized in preliminary tests, several in vitro and in vivo tests were also necessary to confirm the blend’s mildness.
Patch test: To test for skin irritation, a 0.7% active solution of the OF surfactant combination in deionized water was tested. Samples were applied to subjects’ (n = 20) backs and/or forearms in an occlusive manner using aluminium, 20-mL Finn Chambers. The skin irritation potential of the product was visually evaluated at three control times: 30 min (T1) after occlusive application, to evaluate immediate skin irritation potential (ISIP); 48 hr (T2) after occlusive application; and 24 hr after removal (T3), to evaluate skin irritation potential (SIP).
Repeated wash test: To evaluate compatibility with the skin barrier, two detergent formulae were assessed for effects on transepidermal water loss (TEWL), pH and redness of skin after repeated washing. Two solutions were tested, both 14% active concentrations: one solution of SLES and DLS at a ratio of 80:20, and another of SLES and the OF blend, at the same ratio. Instrumental measurements were carried out in a temperature and humidity controlled room, at 24 ± 2°C at 50 ± 10% relative humidity. Volunteers (n = 12) were instructed not to wash their forearms 3 hr before and not to apply any products 12 hr before the basal control measurements.
Assessments were performed on the volar area of each forearm, where three sites measuring 9-cm2 each were delimited: one treated with SLES/DLS at 80:20; one with SLES/OF at 80:20; and one treated with tap water as a control. The assignment of these areas was randomized among the subjects, and the study lasted for five days. On the first day (T0), basal measurements of skin pH, TEWL and skin redness were taken in each area. The test engineer, wearing latex gloves, carried out the washing protocol as follows: 1) washing skin with 1 mL of the product for 60 sec; 2) rinsing with tap water for 30 sec; and 3) gently drying with a towel. The control area was washed with tap water for 90 sec and dried gently with a towel. Washing was repeated four times with 1 hr between washings on the first four days and twice on the fifth day, for 18 washings in total. Final measurements of skin pH, TEWL and skin redness were taken on fifth day, 1 hr after the last cleansing procedure (Tf). The mildness of the blend would be evident if the values at the beginning and end of the test did not vary significantly, and/or by the absence of significant differences between the treated and control areas.11
Het-Cam test: The Hen’s Egg Test-Chorioallantoic Membrane (Het-Cam) method, based on the approach suggested by Luepke in 1985, was also used to test irritation potential. This test analyzes irritant reactions and severity of vascular damage on the chorioallantoic membrane of fertilized chicken eggs, in response to exposure to test samples.12 Here, the authors compared solutions of 7% active SLES, the OF blend, and SLES + the OF blend (at 80:20).
Sensory Assessments
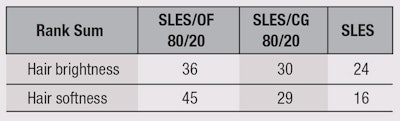
Lastly, sensory evaluations for the effects of samples on hair brightness and softness were conducted using a ranking approach. Three straight, medium-brown, virgin hair tresses were treated with one test product each according to the following procedure: 1) washing each lock with 1 mL of test shampoo for 30 sec; 2) rinsing it with tap water for 1 min; and 3) gently drying with a hair dryer. The same solutions of 7% active SLES, the OF blend, and SLES + the OF blend (at 80:20) used in the Het-Cam test were evaluated.
The three tresses were then presented in a balanced, randomized order to 12 panelists, who visually and tactilely evaluated them with regard to brightness and softness according to the same standard. Each assessor ranked the tresses by increasing degree—first, from bright to brightest, then arranging them from least soft to softest.13, 14 For each attribute, the rank position given by each panelist and the rank sum, obtained by adding the individual rankings, were calculated.
Following are the results obtained from all the different tests, starting with foam properties and thickening.
Results: Foaming
The foaming ability of the new OF blend was very good, as shown in Figure 4. In comparison with all tested surfactants, it provided the highest foam with the best stability; the foam was also compact, homogeneous and stable (not shown). The OF blend also imparted synergistic effects when added at just 20% total active content, as seen in the case of SLES, DLS and CG. The same synergy was obtained in the ternary systems when SLES was the main surfactant at 60% total active content and the OF blend and DLS, DCA or CG were used as secondary surfactants at 20% each. Figure 5 shows that the addition of the OF blend, even at a small percentage, increased both the foam amount and stability.
Interestingly, the new blend performed well in several binary mixtures, too. It also increased the foam generated by other surfactants; in particular, the best synergy was obtained at the ratio of 80:20, where OF was the secondary surfactant. In ternary systems, it was also observed that the addition of small quantities of OF (20% total active) allowed the formation of more compact foams and improved their amount and stability (not shown).
Results: Thickening
As reported, different ingredients were used to verify the thickening capacity of the OF blend—either alone or in combination with other surfactants, and in binary and ternary systems. Initially, it was tested in combination with three different inorganic salts. Figure 6 shows the viscosity behavior of the new blend with increasing amounts of NaCl; results with the other two salts, MgCl2 and CaCl2, are not reported due to compatibility issues—possibly related to the anionic moiety of the blend, which caused significant precipitation. The addition of NaCl gave the best performance between 2.5% and 3.25%.
The performance of other thickening ingredients were also evaluated, the results of which are reported in Figure 7. All tested ingredients thickened the new blend without compatibility issues. PEG-120 methylglucose dioleate, selected to compare the behavior of the OF blend with the surfactants used in the described foam test, was one of the best performing agents. As shown in Figure 8, values were recorded for each ingredient alone, in solution at 14% active and in combination with the OF blend at different ratios. The thickener PEG-120 methylglucose dioleate was also introduced at 2.5%. Note that the viscosity value of OF with 2.5% PEG-120 methylglucose dioleate is not shown as it was, comparatively, too high. The same approach was used for the ternary mixtures; Figure 9 shows all values.
It was evident that OF performed the best, as it was easily thickened. Moreover, it significantly improved the ability of the added surfactants to be thickened, both in binary and ternary systems. The improvements reached 50% of values when OF was added at a ratio of 1:1 in binary systems and between 20% and 50% when added as secondary surfactant in ternary systems. It was particularly interesting to note that SLES, which is difficult to thicken, was easily thickened with a small amount of the OF blend.
Results: Compatibility
In trials to verify the behavior of the OF blend at different pH values and its compatibility with other ingredients, no issues were observed when it was introduced up to a level of 15% in a series of formulations. These included: makeup remover, anti-dandruff shampoo, intimate cleansing, shower baby oil, toothpaste, mouthwash and a cleansing gel. However, a solution containing 14% active OF turned cloudy when it was brought to a pH value below 5.5—note that the starting pH of the solution was ~ 7.00. This was likely caused by the N-acyl-amino-acid anionic chain becoming partially insoluble, and could be overcome when the OF blend was used in combination with other surfactants; here, pH values of ~ 4.00 could be reached. As another option, if the OF blend is the only surfactant, the addition of 5% ethanol or glycols such as butylene glycol enables lower pH values to ~ 5.30.
Results: Patch Test
The patch tests were carried out on 20 volunteers that reported sensitive skin, 19 females and 1 male, of an average age of 43.7 years. Separate assessment for ISIP and SIP considered the intensity of reactions, as well as number of cases of irritation. The OF blend did not induce any reactions related to an ISIP. In the case of SIP, the product induced minimum reactions. No allergic reaction was observed, so according to these results, the new blend shows an irritation potential significantly lower than common surfactants. This has been further confirmed by successive tests (not shown).
Results: Repeated Wash Evaluations
The positive results in the first rounds of tests drove the authors to verify the mildness properties of the new blend with more refined methods. Tests carried out on 12 female volunteers, with an average age of 35.2 years, gave the following results.
TEWL: All washings, including those using SLES/DLS, SLES/OF and water, induced a statistically significant increase in TEWL values. Comparison of the treatments indicated that the SLES/OF blend increased TEWL parameter less, although the differences between SLES/OF and SLES/DLS were not significant. Differences were statistically different from the water-only treatment (see Figure 10).
Skin pH: After repeated washings, a statistically significant increase of the mean values of skin pH was observed in all areas, including those treated with SLES/DLS, SLES/OF or water. Comparison of these results did not show a significant difference, indicating a similar behavior for the two detergents and water (see Figure 11).
Skin redness: The evaluation of skin redness was carried out using a chromameter to capture the a* value (i.e., red-green color axis parameter) of skin. After repeated washings, statistically significant increases in the mean values of skin redness were observed in the areas treated with both SLES/DLS and SLES/OF, although to a lesser extent with the SLES/OF blend. Washings with water did not induce significant increases in skin redness (see Figure 12). These results confirmed the mildness of the new blend was comparable to that of the well-known mild surfactant DLS. It was also evident that mildness of the blend was satisfactory, and that its addition reduced the harshness of SLES. It’s effect on skin was similar to that of tap water—as far as pH and skin redness were concerned.
Results: Het-Cam
Similar to the previous tests, the results of the Het-Cam test (see Figure 13) show the OF blend improved the effect of SLES on the skin. For this test, the Q value gives an indication of how aggressive or mild a substance could be to the mucosa. Thus, the higher the Q value, the more aggressive the substance. The score of 2.1 given to SLES makes it a “strong” irritant; on the contrary, the OF blend ranks at 1.11, putting it in the “slight” irritant range. Further, the addition of the new blend to 20% active SLES downgraded SLES’s ranking simply to “irritant.”
Results: Sensory
Finally, and as a consequence of previous results, the new blend was compared with the reference mild surfactant coco-glucoside in a sensory evaluation. Both ingredients, in mixtures with SLES, were tested to evaluate which could better reduce the defatting activity of SLES, which decreases hair brightness and softness. A solution containing only SLES was used as the control. All three solutions were 14% active. Table 1 shows the rank sums obtained for each sample; the greater the value, the better the performance.
In order to identify how the pairs of samples differ from each other, the critical value of Least Significant Difference (LSD) at a 0.05 α-risk level is calculated as follows:
where M = the number of assessors involved in the test, and N = the number of tested samples. The LSD value is the smallest difference between the rank sums of any pair of samples. This calculation is necessary to conclude that products are significantly different in relation to a selected attribute. In this study, the LSD value was equal to 10.7.
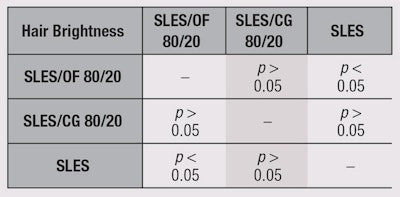
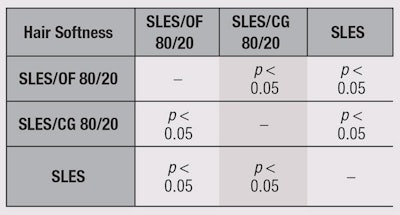
On the basis of this calculated LSD value, at a 0.05 α-risk level, a paired comparison between the rank sums of the samples was performed. Results are shown in Table 2 and Table 3. The pairs of samples whose rank sums differed by a value equal to or greater than 10.7, the LSD value, can be considered significantly different at p < 0.05, with regard to the evaluated sensory features.
The tress treated with the sample SLES/OF performed the best in terms of hair brightness and softness. A statistically significant difference was also evident between the SLES/OF and SLES treatments for both hair brightness and softness. The tress treated with SLES/OF was also perceived as softer and brighter than the tress treated with SLES/CG.
On the basis of these results, it is possible to draw the following conclusions. First, the SLES/OF sample gave the best results in terms of hair brightness and softness. Hair treated with SLES/OF was perceived as being significantly brighter and softer than hair treated with SLES. Finally, hair treated with SLES/OF was perceived softer and brighter than that with SLES/CG.
Conclusion
Foaming capacity, ease of thickening, mildness and good sensory properties are difficult characteristics to achieve with one cleansing ingredient. However, the results achieved with the new OF blend are promising. They suggest that the OF blend is a potential alternative to both traditional surfactants (such as SLS) and mild modern surfactants (including DLS or coco-glucoside), offering improved performance. The capacity to improve the characteristics of associated surfactants with the addition of the blend at just 20% active content is especially interesting. The new OF blend enriches the formulator’s selection of vegetable, eco-friendly and mild surfactants to overcome the functional and sensorial gaps that typically restrict his or her creativity.
References
- L Rigano, G Giammarrusti and F Rastrelli, Vegetable oils–The base of new active principles, SÖFW 132 1-2, 25-33 (2006)
- L Rigano, Vegetal-derived emulsifiers for improved stability and formula efficiency, Cos & Toil 126(8) 578-585 (Aug 2011)
- L Sousselier, Novel ingredients from olive—Nature’s gift for beauty, presented at PCIA, Seoul (Mar 2001) 4. AK Kiritsakis, Virgin olive oil composition and its effect on human health, Inform (13)3 237-41 (2002)
- R Watts and L Sousselier, Fruits of the gods, Soap, Perfumery & Cosmetics 63-8 (Oct 2000)
- VM Verallo-Rowell, KM Dillague and BS Syah-Tjundawan, Novel antibacterial and emollient effects of coconut and virgin olive oils in adult atopic dermatitis, Dermatitis 19(6) 308-15 (Nov/Dec 2008)
- N Mikami and R Oota, An amino acid based surfactant targeting shampoo based on a natural concept, SÖFW 134 1/2 27-32 (2008)
- K Sagawa, Frag J 31(9) 63 (2003)
- E Shiojiri et al, J Soc Cosmet Chem Japan 30(4) (1996)
- H Yoshihara and D Kaneko, Frag J 24 (7) (1996)
- AM Grunewald, M Gloor, W Gehring and P Kleesz, Damage to the skin by repetitive washing, Contact Derm 32 225-232 (1995)
- NP Luepke, Hen’s egg chorioallantoic membrane test for irritation potential, Chem Toxic 23(2) 287-291 (1985)
- Sensory analysis–Methodology–Ranking, International Standard ISO8587:2006 (E) (2006)
- M Meilgaard, GV Civille and BT Carr, Sensory Evaluation Techniques, 2nd ed, CRC Press Inc, Boca Raton, FL (1991)
- M Meilgaard, GV Civille and BT Carr, Sensory Evaluation Techniques, 2nd ed, CRC Press Inc, Boca Raton, FL (1991)