Image by Symbiot at Adobe Stock
The European Commission’s July 8, 2025, reform proposal for Regulation (EC) No 1223/2009 aims to modernize and streamline the cosmetics legal framework. BELAB Services reports this draft proposal, "responds to growing concerns in the cosmetics sector regarding the interpretive rigidity of the current legal framework and the obstacles it poses to innovation."
Key Proposed Amendments
BELAB outlined the following proposed amendments:
- Article 14a clarifies procedures to add colorants, preservatives and UV filters to the regulation's annexes. CosLaw.EU noted, "This amendment provides greater legal clarity for applicants and is expected to foster innovation in the industry."
- Article 15, related to CMR substances, clarifies that their prohibition will only apply to dermal exposure hazards; those posing risks through ingestion or inhalation will not automatically be excluded from cosmetics.
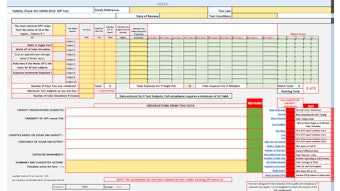
- In addition, the prior notification of nanomaterials required by Article 16 will be removed. Instead, relevant data will be included in Part A of the Cosmetic Product Safety Report.
CosLaw.EU explained: "Cosmetic products containing nanomaterials should not be considered less safe than other cosmetic products, as they undergo the appropriate safety assessment under the responsibility of the Responsible Person."
- The revision of Article 22 removes the requirement that Member States review and report on their market surveillance activities every four years — which is redundant since the Information and Communication System on Market Surveillance (ICSMS) system enables real-time sharing of product data.
- The glossary of common ingredient names under Article 33 will be discontinued. Labels will instead rely on International Nomenclature Cosmetic Ingredient (INCI) terminology, which will be reflected throughout the whole Cosmetics Regulation.
- Lastly, as noted, Annex I has been revised to require Part A of the Cosmetic Product Safety Report to include "the specification of nanomaterials present in the formula, including their chemical names (IUPAC) and other descriptors as specified in the preamble to Annexes II to VI, size of particles, physical and chemical properties."
Next Steps
The proposal will undergo legislative scrutiny by the European Parliament and Council of the EU, CosLaw.EU reports.