
Washing hands with soap and water, and/or use of hand sanitizer with at least 60% alcohol or alcohol-based hand rubs (ABHR) are recommended to help prevent the spread of disease during the COVID-19 pandemic.1 At the start of the pandemic, as is well-known, there was high demand for ABHRs, resulting in a shortage.2 In response, the U.S. Food and Drug Administration (FDA) issued temporary policies to allow for additional manufacturing of ABHRs. These policies were withdrawn on Dec. 31, 2021, however, and all manufacturers of ABHRs must now conform to the regulatory requirements established in the FDA’s 1994 Tentative Final Monograph for Healthcare Antiseptic Drug Products (TFM), showing that hand sanitizing products are safe and effective.4
With the current focus on good hand hygiene, increased handwashing practices and ABHR use, it is anticipated that there will be an increase in skin irritation or dermatitis.3 The personal care product industry has responded to this rising concern by offering products with additional moisturizing claims. ABHRs enhanced with moisturizers may help to improve or maintain skin moisture and prevent the skin from drying. This addition may also help alleviate any damaging or drying effects on the condition of the skin due to the increased use of sanitizing products.3 These products, which include more than one regulated claim on their label and in marketing, reflect a rising trend within the personal care product industry for providing consumers with more complex products.
See archived: A Safety, Toxicity and Irritation Testing Primer
Increasing Functions = Increased Data Requirements
Multifunctional products like moisturizing ABHRs may offer solutions to unforeseen challenges consumers face due to a rapid shift in their personal care habits. Yet, as the needs of consumers continue to grow more complex, further challenges and considerations arise for the manufacturers of these same products. These challenges can be linked to the emerging industry practice of placing historically separate product claims on the same product label.
For example, moisturizers are categorized as cosmetic products whose regulatory guidance is more open-ended and less precise about required testing.6 And, as stated, companies are adding moisturizing claims to hand sanitizer products, which are required to meet the efficacy requirements outlined by the final monograph previously mentioned. Therefore, companies now have a product for which they must provide efficacy data for two distinctly regulated claims: antimicrobial efficacy and skin moisturization.
The hand sanitizer claim requires companies to demonstrate significant reduction (2-3 log) of a microorganism population while the moisturizer claim requires supporting data showing an increase in skin moisture compared with a control. While efficacy criteria for the claims remain relatively unchanged, the combination of claims presented on a single label elevates the data requirements for these products.
Additional uncertainties regarding the efficacy and safety criteria also result from the wide variety of possible active ingredient combinations. Historically, this issue has been shown with the combined use of certain products. For example, considerable data exists showing that certain lotions can inhibit the effect of chlorhexidine-containing antimicrobial hand washes.5 This example highlights the importance of appropriate efficacy data when combining various claims, especially when combining multiple active ingredients on a singular label.
The inconsistency of the amount of data available for the plethora of possible active and inactive ingredients found in multifunctional products makes it difficult for a one-size-fits-all policy regarding efficacy and safety data.
See archived: Back to Basics, Part III; Testing for Preservation and Stability
Study Design for Multifunctional Products
The question, then, remains: How does a personal care product manufacturer ensure they are conducting the appropriate testing for their multifunctional product? The answer can be found by dissecting what elements of the product require new data. For starters, the efficacy data for each claim must be supported as if each claim was present on a separate product. The manufacturer must then determine the regulatory requirements for each claim. As per the prior example, a moisturizing hand sanitizer must abide by the tentative final monograph for antimicrobial efficacy as well as demonstrate data supporting the moisturizing effect of their product. This remains true for personal care products regardless of formulation.
In addition to efficacy, safety also remains a crucial requirement requiring appropriate data. Ensuring a product does not cause irritation or harm to consumers is vitally important, and generally required by many regulatory agencies. Finally, the new point of consideration with multifunctional products is that of active ingredient interference. Like the chlorhexidine example given, ensuring the combination of actives does not have efficacy-mitigating effects must be evaluated when developing new products.
Breaking this down, the three main areas of consideration for evaluating the data requirements for multifunctional products are: efficacy, safety and ingredient interactions (see Table 1).
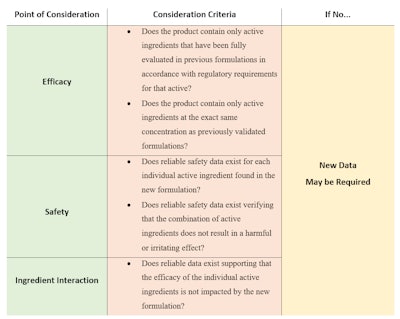
Narrowed to three main points, approaching the question of study design becomes more manageable. Starting with efficacy, analyzing the product through a scientific and rational scope can assist in determining what data may need be to be generated. For example, if a product is an amalgamation of two existing products from the manufacturers’ current line, each original active has significant efficacy data associated with it, and the interaction between the actives is known, then it is possible that no additional efficacy data for each active ingredient is required.
In contrast, if a manufacturer is looking to add a newer, less-studied active ingredient to its product formulation, an appropriate efficacy evaluation of that active ingredient may be necessary. As a general principle, treating each claim as separate efficacy requirements simplifies the decision as to what data is required.
Beyond individual efficacy claims, safety and potential ingredient interactions must also be taken into consideration. While these areas may appear to be complicated, they offer an opportunity to efficiently design a study that encapsulates two or more of the main areas of consideration. Such an example might be a clinical in-use study that analyzes the product in a replicated environment of its intended real-life use. By evaluating the product in this environment, measurements can be taken that support both the safety and efficacy of the final formulation of the product.
See archived: Stability Testing Guidance for Product Safety and Shelf-light Insight
It is important to understand that the appropriate design of a study such as this will be critical for ensuring the correct data is collected. As such, partnering with an experienced laboratory with an in-depth understanding of both regulatory requirements and clinical study design could be useful, especially considering the study design can vary tremendously between products.
Moisturizing hand sanitizers are just the tip of the iceberg, as companies will continue to offer increasingly complex products from ever-diversifying personal care product lines. Breaking down the data requirements into the three distinct components listed can be a powerful tool to assist in navigating data uncertainties and decision-making as these products grow more complex. It is always strongly recommended, however, that either an experienced laboratory or the appropriate governing agency be consulted to ensure all data requirements are being met.
Conclusion
Unforeseen global events and technological developments will continue to define the landscape in which organizations in the personal care product industry continue to conduct business and meet the needs of their markets. As products become more complex and evolve in response to these changes, the approach of ensuring the safety and well-being of consumers must grow as well.
As new multifunctional products are developed, evaluations that reflect their real-life use will become imperative to ensure consumer safety and satisfaction. Through studies designed via an ethical and analytical lens, in accordance with the active ingredients’ relative history, appropriate data can be generated for multifunctional products regardless of the relative complexity.
References
1. Centers for Disease Control and Prevention (2021, Aug 10). When and how to wash your hands. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/handwashing/when-how-handwashing.html
2. Nytimes.com (2022, Mar 11). Coronavirus has caused a hand sanitizer shortage. What should you do? Available at https://www.nytimes.com/2020/03/11/smarter-living/wirecutter/coronavirus-hand-sanitizer.html





!['We believe [Byome Derma] will redefine how products are tested, recommended and marketed, moving the industry away from intuition or influence, toward evidence-based personalization.' Pictured: Byome Labs Team](https://img.cosmeticsandtoiletries.com/mindful/allured/workspaces/default/uploads/2025/08/byome-labs-group-photo.AKivj2669s.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&fp-x=0.49&fp-y=0.5&fp-z=1&h=191&q=70&w=340)




