Skin chronological aging, also called intrinsic aging, has been characterized on a microscopic level by reductions in total collagen content, elastin synthesis1 and hyaluronic acid content. These reductions are due, in part, to the cells involved becoming senescent and losing their synthesis capabilities.2 Decreases in these skin components further lead to decreased skin thickness, modifications of skin anisotropy, and the loss of skin firmness and elasticity, which are visible at the macroscopic level.
Numerous skin treatments and dermatological procedures, ranging from noninvasive to invasive, are available to promote youthful skin. Such treatments include topical retinoids, glycolic acid, botulinum neurotoxin, soft tissue fillers,3 lasers,4 radio frequency, microneedle stimulation, surgical procedures, endocrine therapies and mesotherapy.5 Over the last decade, interest has increased for minimally invasive techniques, particularly for facial rejuvenation. For example, the injection of fillers6 such as collagen and hyaluronic acid into soft tissue has more frequently been performed.
The ideal dermal filler should offer long-lasting aesthetic improvement with good biocompatibility, and therefore cause minimal side effects. However, all dermal fillers induce transient adverse effects—including pain, discomfort, redness, bruising and swelling—and can sometimes even induce serious and potentially long-lasting adverse effects such as granuloma and infections.7–10 Currently, the major dermal fillers used are based on hyaluronic acid, but since their filling effects are transient, lasting for only six to nine months,3, 11, 12 patients must undergo multiple time-consuming and expensive injection sessions to maintain satisfactory effects.12, 13 On the other hand, collagen-based fillers last no longer in the skin than hyaluronic acid products, and also present drawbacks due to their animal origin—such as the risk of virus and/or bacterial contamination from animals to humans. They also can induce significant allergic reactions due to their protein content.14, 15
As a safe and effective alternative to the described facial rejuvenation techniques, the authors developed an active ingredient to target the reactivation of senescent fibroblasts by stimulating their metabolic pathways. During this process, an assumption was made that, in order to counter the aging process, aged fibroblasts must be reactivated to produce proteins impacted by aging. These proteins include pro-collagen I, collagen types I and III, and elastin. The active ingredient developed therefore consisted of materials that act upon these proteins.
Methyl-glucoside-6-phosphate was incorporated as a cellular source of energy. This ingredient is a mimetic of glucose-6-phosphate—a glucose sugar phosphorylated on carbon 6. This compound is common in cells, as the vast majority of glucose entering a cell will become phosphorylated in this way. Glucose-6-phosphate has many possible fates within the cell since it lies at the start of two major metabolic routes, including glycolysis and the pentose phosphate pathway.
Proline and lysine, two essential amino acids for the synthesis of collagen,16 also were included, in addition to elastin and copper—to maintain the activity of lysyl oxidase. Lysyl oxidase is an enzyme involved in the extracellular maturation of collagen and elastin.17 Specifically, it is responsible for the formation of lysine-derived cross-links in connective tissue.18 Normal cross-linking is essential to providing resistance to elastolysis and collagenolysis by nonspecific proteinases.
The biological activity of the resulting ingredient was then investigated using in vitro models, ex vivo explants and human volunteers, as described here.
Materials and Methods
Composition, concentration of the active: The active ingredient developeda is a 50% w/w water and glycerin solution containing α-D-methyl-glucopyranoside-6-phosphate (200 mM), in ionic combination with the essential amino acids proline (100 mM) and lysine (90 mM), and copper (20 mM). The concentration of active ingredient used was adapted depending on the biological model used. For this purpose, the ingredient was diluted in deionized water. For in vitro studies, the active was used at 0.1%, 0.5% and 2% w/w final concentrations; ex vivo, the active was used at 0.5% w/w. Note that in both in vitro and ex vivo studies, the active was tested without glycerol.
For the in vivo study on volunteers, the active was incorporated at 1% w/w in a cream also comprising: water (aqua), octyldodecyl neopentanoate, octyldodecyl, octyldodecanol, myristyl myristate, acrylates/C10-30 alkyl acrylate crosspolymer, sodium hydroxide, phenoxyethanol, methylparaben, ethylparaben, butylparaben, propylparaben and isobutylparaben.
Safety assessments: Safety tests were performed using: the Ames test, to assess mutagenicity; a human reconstructed cornea model, to determine ocular irritation; and patch tests on human volunteers, to determine hypersensitization. Evaluations were performed according to the recommendations of European Commission (EC) Regulation No. 1223/2009 on cosmetic products and EC Regulation No. 1907/2006 concerning the registration, evaluation, authorization and restriction of chemicals (REACH), which refers to environmental safety.
In vitro test cells: Young and aged primary human fibroblastsb were used for in vitro tests. Aged fibroblasts were obtained by passaging young fibroblasts several times (17th) according to the Hayflick model.19
MTT viability assessment: Fibroblasts cells were incubated for 72 hr in a culture mediumb consisting of a low glucose concentration of 1 g/L with increasing amounts of the active ingredient—i.e., 0.08%, 0.4% and 2% w/w. After treatment, cells were incubated with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reduced in blue formazan crystals by succinate dehydrogenase. After cell dissociation and formazan crystal solubilization using dimethyl sulfoxide, the optical density of the extracts at 540 nm, proportional to the number of living cells and their metabolic activity, was recorded with a microtiter plate reader. Morphological observations of cells were performed under a microscope.
ELISA assessment on pro-collagen-I synthesis: Young and aged fibroblasts were seeded in 96-well plates and incubated for 24 hr in culture medium. The medium was then removed and replaced by an assay medium containing glucose at the concentration of 4.5 g/L under the following conditions: without any supplement (control); with the active ingredient at concentrations of 0.1%, 0.5% and 2% w/w, respectively (assays); or with the mix of references compounds including 10 ng/mL of TGF-β and 20 µg/mL of vitamin C (positive test validation). The cells were incubated for 72 hr, and all experiments were performed in triplicate. After incubation, the supernatants were collected to quantify pro-collagen I synthesis using a pro-collagen I ELISA kitc.
Ex vivo test protocol: Aged skin explants from a 74-year-old Caucasian male were obtained from abdominoplasty. Each explant had an average diameter of 11 mm. The explants were kept in a survival culture mediumd at 37°C in a humid, 5% CO2 atmosphere. Explants either were left untreated (n = 9) for 0, 6 or 11 days, or treated (n = 6) for 6 or 11 days with 0.5% w/w of the active ingredient. The active ingredient, diluted with only water, was applied to the surface of the explants on days 0, 1, 4, 6 and 8 in the aforementioned concentrations on the basis of 2 mg/cm2.
Preparing explants for histological analysis: After treatment, explants were cut and frozen at -80°C. Samples fixed in formalin or Bouin were dehydrated and embedded in paraffin; 5-µm thick sections were made using a microtomee, and the sections were mounted on glass slidesf. The frozen samples were cut into 7-µm thick sections using a cryostatg. Sections were mounted on silanized glass slidesh and microscopical observations were made using a microscopej.
Collagen, tropoelastin and elastin immunostaining: The immunostaining of collagen types I and III, tropoelastin and elastin was performed on frozen sections using the following specific antibodies: anti-collagen I rabbit polyclonal antibodyk diluted at 1/200; anti-collagen III goat polyclonal antibodym diluted at 1/100; anti-tropoelastin mouse antibodyn diluted at 1/25; and anti-elastin rabbit polyclonal antibodyp diluted at 1/800. All immunostainings were assessed by microscopic observation and image analysis using software. Analyses were performed on approximately 9–13 images for each batch, based on the detection of staining by intensity level selection and surface measurement of the region of interest.
In vivo Studies
Twenty women between the ages of 54 and 68, having fine or visible expression lines on their faces and no dermatological issues, were recruited. Subjects agreed to not use other topical products during the study. The volunteers applied a placebo cream to one side of their face and the same cream containing 1% active ingredient to the other side of their face twice daily for 60 days. Various measurements were recorded at D0, D15 and D60.
Collagen density assessment: A digital epiluminescence microscopy system designed for taking shadow-free magnified images of skinq was used to measure and image the amount of collagen present in the dermal layers of skin. This instrument consists of a handheld probe containing light sources and a digital imaging sensor. Light from the controlled illumination source within the probe shines on an area of skin through a window at the end of the probe, placed in contact with the skin surface. The measurements recorded consist of several images taken in quick succession.
Instrument-specific software then performed calculations of the different-wavelength images using information about the layered nature of skin tissue and its spectral characteristics. The instrument produces images for melanin, blood and collagen, which are then used for analysis. This system was used to track changes in the organization of collagen fibers at T0, T15, T30 and T60. Images of each of the sites were captured using the instrument, and the results are expressed by the mean of light received by the tool. The quantity of light received is inversely proportional to collagen density, so greater quantities of light indicate less collagen density.
Anisotropy index: Aging processes in skin change its natural organization and alignment. For younger individuals, lines are homogeneously isotropic—i.e., evenly oriented in all directions—but with age, furrows gradually appear, giving rise to persistent deep furrows and forming highly oriented wrinkles. The skin’s anisotropic index reflects the state of skin in micro-relief, allowing for the assessment of a product’s effects on skin surface topography.20 The measurements made here were performed using an imaging devicer whose index was calculated from the evaluation of grayscale images at each study time.
Firmness and elasticity: The mechanical properties of skin indicate the functional state of tissues, such as the elasticity of fibers, the curvature of connective bundles and wrinkles of the stratum corneum. The described study was performed using a cutometers. The measuring principle was based on the suction method; negative pressure was created in the device and skin was drawn into the 2-mm diameter cylindrical aperture of the probe. Inside the probe, the penetration depth was determined by an optical measuring system. Each suction phase was followed by a relaxation phase. The study protocol cycle lasted four seconds in total; suction = 2 sec, and relaxation = 2 sec. The negative pressure applied was 500 millibars, and the area measured was on the cheekbone.
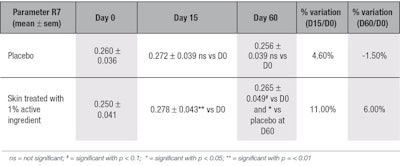
During the suction phase, the deformation of skin by the negative pressure first indicates elastic resistance, followed by the viscous nature of skin’s carbohydrate and water components; taken together, these represent skin firmness. During the relaxation phase, the immediate recovery of skin measures its total cutaneous elasticity, whereas the delayed return of skin to its initial position measures its visco-elasticity. This parameter, referred to as R7, was recorded to compare changes in skin firmness and elasticity before and after treatment. The closer the value is to 1, the higher its elasticity and firmness.
Anti-wrinkle activity via fringe projection: The anti-wrinkle activity of a cosmetic product was assessed by fringe projection on a silicone replica. Wrinkles on the replica were analyzed in the same location for each time point. A picture of each replica was taken with a fringe projection profilometert and transferred to softwareu for assessment. The parameters measured were depth and volume of wrinkles.
The principle of the measurement is based on profilometry by fringe projection interference and analysis by software. The surface of the three-dimensional object, the silicone replica in this case, is described by an equation with three unknowns—x, y and z. The relief of the replica is calculated as an f(x, y) function, describing the height, z, of point M on the surface; thus z = f(x,y). In order to obtain several curves of z = Zi height, the object was lit with a halogen fringe projector with a 6-mm (-3 mm < z < 3 mm) depth of field using a 25× objective lens. All calculations were made using the data acquisition and processing software, which enables the calculation of the average depth and volume of wrinkles.
Results of the effects of a cosmetic product on the evolution of the various parameters are expressed in μm for the average depth, and mm3 for the average volume. Calculations of the overall effect were performed by determining the variation percentage compared to the initial measurement for each parameter. Note that statistical analyses were performed on all results using the Student’s t-test on paired data.21
In vitro, Ex vivo Results and Discussion
Safety assessments: Assessments of the 100% pure ingredient using the Ames test, a human reconstructed cornea model and human volunteers indicated it has good tolerance for skin. The ingredient was therefore qualified as non-mutagenic, a non-irritant, and non-sensitizing for human skin.
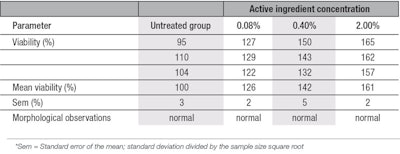
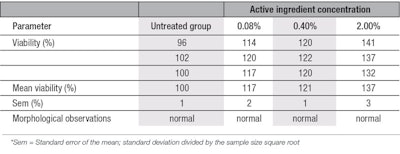
MTT viability assessment: The MTT test demonstrated non-cytotoxicity of the active ingredient on both young and aged fibroblasts, and no cell death was observed morphologically at a concentration of up to 2% w/w—the highest concentration evaluated. Tests also demonstrated the ingredient stimulated the viability (proliferation) of both young and aged cell types; viabilities increased with 2% of the active, in comparison with the untreated group. In young fibroblasts, cell viability improved by 26%, 42% and 61%, respectively with 0.08%, 0.40% and 2.0% of the ingredient (see Table 1). In aged fibroblasts, the improvement of cell viability was 17%, 21% and 36% at the same respective concentrations (see Table 2). The cell viability improvement was more pronounced in young cells due, likely, to the fact that they have more efficient metabolism than aged cells.22, 23
ELISA assessment on pro-collagen-I synthesis: The 2% active ingredient significantly stimulated pro-collagen-I synthesis in both young and senescent fibroblasts (see Figure 1). However, compared with the respective controls, its effects were stronger in aged fibroblasts, which showed a 47% increase in pro-collagen-I synthesis, than in young fibroblasts, yielding a 16% increase. This may be explained by the fact that young fibroblasts use the α-D-methyl-glucoside-6-phosphate within the active as a source of energy; i.e., in the glycolysis pathway and Krebs cycle to generate up to 31 adenosine triphosphate (ATP) molecules for their cellular division.24
In contrast, the aged fibroblasts use the proline and lysine amino acids, the copper, and the methyl-glucose-6-phosphate from the active to reactivate their collagen and protein synthesis. Proline and lysine are two key amino acids in collagen. As noted, copper was included in the active as a cofactor for the enzyme lysyl oxidase, and lysyl oxidase catalyses the post-translational oxidation of certain lysine and hydroxylysine residues in elastin and collagen.25
These hypotheses are consistent with data published by Zwerschke et al.,26 who studied changes in energy metabolism associated with cellular senescence via the determination of glucose consumption by young and senescent human dermal fibroblasts. The researchers found a significant increase in the consumption of glucose in senescent cells but its uptake was not used in the glycolysis pathway; it was used for amino acid production instead, especially for alanine. They also showed that only a minor proportion of glucose was converted into lactate in senescent cells, demonstrating that most of the input glucose was used for other purposes in senescent human dermal fibroblasts. Other authors have shown a deficiency in glucose-6-phosphate dehydrogenase activity in senescent cells,26, 27 thus explicating the non-use of glucose-6-phosphate in the glycolysis pathway.
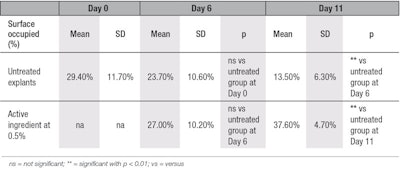
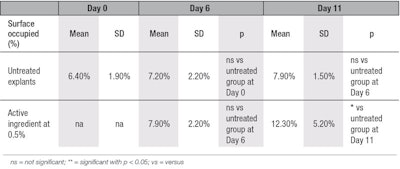
Collagen immunostaining: In the present study, clear stimulation of collagen type I synthesis was observed in the aged skin explants topically treated with 0.5% of the active ingredient, compared with untreated explants at D6 and D11 (see Figure 2 and Table 3). No staining was observed after replacing the primary antibody by phosphate-buffered saline (PBS), showing there was no aspecific staining. In comparison with D0, at D6, for both young and aged conditions, collagen type I content was decreased (see Figure 3). This reduction in collagen content was likely the result of collagenase activities in the skin explant. Nevertheless, at D6, the decrease in collagen type I content was less pronounced in explants treated by the active ingredient, compared with untreated explants.
At D11, the synthesis of collagen type I was increased by 179% (see Table 3) in presence of the active ingredient, compared with untreated explant at D11. The active ingredient seems to require at least 11 days to counteract the aging processes in these culture conditions. In the untreated explants, from D0 to D11, the aging process was observed through the decrease of collagen type I content over that time.
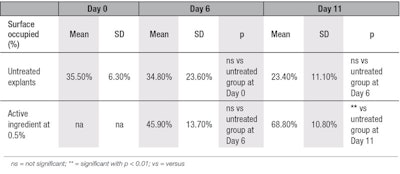
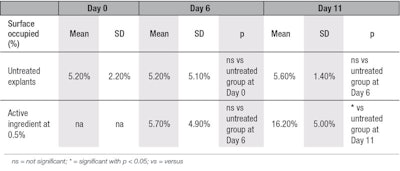
For collagen type III, at D11, a statistically significant 192% increase in its synthesis was observed in the treated conditions versus the untreated ones (see Figure 4 and Figure 5, and Table 4). Again, no staining was observed after replacing the primary antibody by PBS, showing there was no aspecific staining. Thus, these ex vivo experiments demonstrate the effectiveness of the active to induce the stimulation of pro-collagen I, collagen I and collagen III synthesis by aged skin fibroblasts after treatment, whereas it is well-known that in aged cells, the synthesis of these entities decreases.28
Tropoelastin and elastin immunostaining: Elastin is produced during the early years of life in low levels; however, its half-life has been described to be around 74 years29—the longest-lasting protein in the human body. This natural low level of elastin may mean that damage caused to it cannot be efficiently repaired without exogenous treatment, and that skin will gradually lose its elasticity.30 Interestingly, application of the active on skin explants stimulated both tropoelastin (see Table 5 and Figure 6) and elastin synthesis (Table 6 and Figure 7); compared with untreated explant at D11, the active ingredient statistically increased the synthesis of tropoelastin by 56% and elastin by 189%. Also, once synthesized, the elastin and tropoelastin were deposited directly on the elastic fibers in candelabra shapes, as shown in Figure 8.
In vivo Results and Discussion
With chronological skin aging, wrinkles appear on the face due to variations including: decreases in collagen density, i.e., skin thinning;31, 32 an increase in skin anisotropy, changing the skin micro-relief;20, 33 and decreases in skin firmness and elasticity, i.e., skin sagging.31, 34 As noted, to assess the biological efficacy of the active ingredient in vivo, a clinical test was conducted with 20 women, ages 61 ± 7 years, during the course of two months.
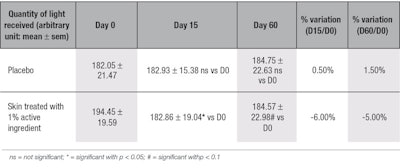
Collagen density: Dermis thickness is known to be directly correlated with the synthesis of new collagen and other dermis components, and measurements showed that the active ingredient improved collagen density by 6% after 15 days, as seen by a decrease in the quantity of light reflected from the skin and read by the devicer; equal results also were observed after 60 days (see Table 7), proving a long-lasting skin-thickening effect. These results were consistent with the ex vivo data showing the active stimulated the synthesis of collagen types I and III.
Data on skin-thickening effects after collagen injections is limited; most literature describes the effects of hyaluronic acid injections. Indeed, Baspeyras et al.35 described a physical increase in dermis thickness—due to the swelling properties of hyaluronic acid—at one month (3.4%) and three months (4.0%), in comparison with day 0, after a hyaluronic acid multi-injection treatment. Nevertheless, careful examination of the results indicates this increase was not significantly different from those obtained with the placebo. Other clinical studies36-38 using histology, cutometry or echography to assess the effectiveness of hyaluronic acid microinjections, failed to show changes in dermis thickness.
Here, in vivo results showed the active ingredient was more efficient than both hyaluronic acid-based fillers and multi-session laser treatments because the stimulatory effect on collagen synthesis was stronger, by 6%, and faster, requiring only 15 days of daily topical treatment. Moreover, the use of the active ingredient showed no drawbacks such as swelling from the injection procedure, as confirmed by a dermatologist.
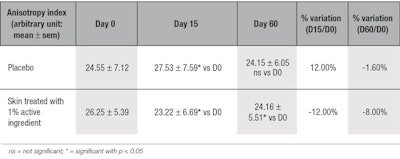
Anisotropy index: The active ingredient significantly improved skin’s micro-relief by reducing the anisotropy of the skin after 15 days, by 12%, and through 60 days, by 8% (see Table 8). These results showed that the skin of the volunteers was not only gaining collagen content, but that the collagen network was reinforced and reorganized with time.
Firmness and elasticity: The data shows that the active significantly improved skin firmness and elasticity by 11% after 15 days of treatment; it also significantly improved firmness and elasticity in comparison with the placebo at D60 (see Table 9). This increase of skin firmness can be correlated with the higher density of collagen in the skin, and to the ex vivo results on collagens type I and III. In parallel, the improvement in skin elasticity is confirmed by the observed stimulatory effect of the active on the synthesis of elastin and its accumulation on the elastic fibers.
In relation, while Baspeyras et al.35 showed that multiple injections of non-cross-linked hyaluronic acid may improve firmness and elasticity, in comparison with D0, no significant difference was observed after three months by measurements made with a cutometer. Reuther et al. and Amin et al.37, 38 also showed an increase in skin elasticity after a hyaluronic acid injection treatment, but no effect on skin thickness was observed.
A long-term clinical study over 24 weeks39 assessed the effect of a daily topical estrogen treatment on the elasticity of photo-aged skin by cutometer, but these results also showed no significant improvement in the skin elasticity after treatment. The efficacy of the tested active ingredient to improve skin firmness and elasticity was therefore better than any observed during mesotherapy procedure or after topically applied estrogen.
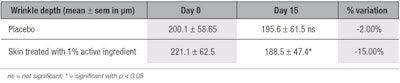
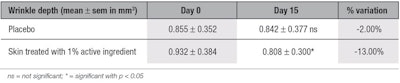
Anti-wrinkle activity via fringe projection: The topical application of the active ingredient significantly decreased the depth of wrinkles by 15% and the volume of wrinkles by 13% (see Table 10 and Table 11, respectively) at D15, in comparison with the placebo. The skin surface was smoothed after two weeks of application of the ingredient, as shown by surface analysis (see Figure 9, side B); no improvement of the skin was observed with the placebo (side A), demonstrating the effectiveness of the active ingredient. The simultaneous decrease of both wrinkle volume and depth achieved with the active suggests that it synthesized new collagen types in the skin, refilling the wrinkles without stretching them. The observed smoothing effect of the skin surface may be the result of the higher content in elastin in skin.
The American Society of Plastic Surgeons40 has stated that a dermal filler injection containing cells obtained from human skin biopsy can decrease wrinkle depth by about 15%, with a minimum of three injections recommended at two-week intervals. The second injection is said to reduce wrinkles by up to 35%, and the third, by 70%. The results described here, however, with the topical active are equivalent to the results of the first injection, i.e., a 15% reduction in wrinkle depth without invasive procedure.
Conclusions
Combinations of different minimally invasive approaches such as botulinum toxin type A, dermal fillers and chemical peels are often necessary to provide efficient, visible facial rejuvenation.41-43 However, this study has shown that age-related decreases in collagen and elastin synthesis are reversible. The tested active ingredient based on a glucose-6-phosphate derivative (methyl-glucoside-6-phosphate), in ionic combination with proline, lysine and copper, can be used to reactivate the synthesis of key extracellular polymers in mature skin. This study also demonstrated the ingredient to be safe and efficient—providing rejuvenation results in two weeks with only simple topical application twice a day.
Cellular and explant results demonstrated an increase in pro-collagen type I, collagens type I and III, tropoelastin and elastin synthesis, which was assumed to result from the assimilation by skin cells of a source of energy and the essential amino acids proline and lysine contained in the tested ingredient. In addition, the copper ion was expected to maintain the activity of the copper-dependent lysyl oxidase enzyme, which was maturing collagen and elastin precursors into reticulated ones in the extracellular matrix.
Finally, topical application of a cream with 1% of the ingredient on the faces of 20 women showed fast and statistically significant results in two weeks, in comparison with a placebo and results at D0. These in vivo results included a reduction in wrinkle depth, improvement of elasticity and thickening of the skin, and were stable from D15 to the end of the clinical investigation at D60. The ingredient first improved skin texture, then maintained activity over time, compensating for natural aging processes.
The present study has demonstrated that topical treatments can deliver efficient results to rejuvenate the skin structure and appearance—results similar to semi-invasive procedures but without side effects. The ingredient is a good example of a newer category of active cosmetic ingredients (ACI), which are the cosmetic equivalent of active pharmaceutical ingredients (API). ACIs arise from the rational analysis of cellular mechanisms. In this case, they open the door to a new generation of “needle-free” cosmetic products. As an outcome, a new category of products will soon rise on the market.
Acknowledgements: The authors wish to acknowledge the funding for this work was provided by Induchem Company.
References
- LB Sandberg, NT Soskel and JG Leslie, Elastin structure, biosynthesis and relation to disease states, N Engl J Med 304(10) 566-79 (1981)
- J Varani et al, Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation, Am J Pathol 168(6) 1861-8 (2006)
- J Carruthers et al, The science and art of dermal fillers for soft-tissue augmentation, J Drugs Dermatol 8(4) 335-50 (2009)
- D Helbig and U Paasch, Molecular changes during skin aging and wound healing after fractional ablative photothermolysis, Skin Res Technol 17(1) 119-28 (2011)
- PG Sator, Skin treatments and dermatological procedures to promote youthful skin, Clin Interv Aging 1(1) 51-6 (2006)
- DW Buck, 2nd, M Alam and JY Kim, Injectable fillers for facial rejuvenation: A review, J Plast Reconstr Aesthet Surg 62(1)11-8 (2009)
- L Requena, Adverse reactions to injectable soft tissue fillers, J Am Acad Dermatol 64(1) 1-34; quiz 35-6 (2011)
- E Gilbert et al, The basic science of dermal fillers: Past and present, Part II: Adverse effects, J Drugs Dermatol 11(9) 1069-77 (2012)
- G Lemperle, V Morhenn and U Charrier, Human histology and persistence of various injectable filler substances for soft tissue augmentation, Aesthetic Plast Surg 27(5) 354-66; discussion 367 (2003)
- DW Kim et al, Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management, J Plast Reconstr Aesthet Surg 64(12) 1590-5 (2011)
- V Turlier et al, Association between collagen production and mechanical stretching in dermal extracellular matrix: In vivo effect of cross-linked hyaluronic acid filler. A randomized, placebo-controlled study, J Dermatol Sci 69(3) 187-94 (2013)
- L Smith and K Cockerham, Hyaluronic acid dermal fillers: Can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration? Patient Prefer Adherence 5:133-9 (2011)
- SR Smith, et al, Duration of wrinkle correction following repeat treatment with Juvederm hyaluronic acid fillers, Arch Dermatol Res 302(10) 757-62 (2010)
- S Brongo et al, Keratoacanthoma arising after site injection infection of cosmetic collagen filler, Int J Surg Case Rep 4(4) 429-31 (2013)
- K Cockerham and VJ Hsu, Collagen-based dermal fillers: Past, present, future, Facial Plast Surg 25(2) 106-13 (2009)
- Kivirikko et al, Enzymatic hydroxylation of proline and lysine in protocollagen, Biochemistry 57 782-788 (Jan 1967)
- N Romero, D Tinker, D Hyde and RB Rucker, Role of plasma and serum proteases in the degradation of elastin, Arch Biochem Biophys 244 161–8 (1989)
- RB Rucker et al, Copper, lysyl oxidase and extracellular matrix protein cross-linking, Am J Clin Nutr 67(5 Suppl) 996S-1002S (May 1998)
- L Hayflick and PS Moorhead, The serial cultivation of human diploid cell strains, Exp Cell Res 25: 585-621 (1961)
- JM Lagarde, C Rouvrais and D Black, Topography and anisotropy of the skin surface with aging, Skin Res Technol 11(2) 110-9 (2005)
- Student, The probable error of a mean, Biometrika 6(1) 1-25 (1908)
- M-CM Odile Damour and P Rousselle, Le vieillissement cutané: La peau de 7 à 77 ans, Ed Media Flash, Paris (1998)
- CK Lumpkin, Jr, et al, Existence of high abundance antiproliferative mRNA’s in senescent human diploid fibroblasts, Science 232(4748) 393-5 (1986)
- LG Boros, WN Lee and VL Go, A metabolic hypothesis of cell growth and death in pancreatic cancer, Pancreas 24(1) 26-33 (2002)
- ED Harris et al, Copper and the synthesis of elastin and collagen, Ciba Found Symp 79 163-82 (1980)
- MS Zwerschke Werner, P Stockl, E Hutter, E Eigenbrodt and P Jansen-Durr, Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence, Biochem J 376 403-411 (2003)
- ML Cheng et al, Glucose-6-phosphate dehydrogenase-deficient cells show an increased propensity for oxidant-induced senescence, Free Radic Biol Med 36(5) 580-91 (2004)
- K Takeda, A Gosiewska and B Peterkofsky, Similar, but not identical, modulation of expression of extracellular matrix components during in vitro and in vivo aging of human skin fibroblasts, J Cell Physiol 153(3) 450-9 (1992)
- SD Shapiro et al., Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon, J Clin Invest 87(5) 1828-34 (1991)
- PC Durai et al, Aging in elderly: Chronological versus photoaging, Indian J Dermatol 57(5) 343-52 (2012)
- A Firooz et al, Variation of biophysical parameters of the skin with age, gender, and body region, Scientific World Journal 386936 (2012)
- W Chen et al, Gender aspects in skin diseases, J Eur Acad Dermatol Venereol 24(12) 1378-85 (2010)
- C Pailler-Mattei et al, In vivo skin biophysical behavior and surface topography as a function of aging, J Mech Behav Biomed Mater (2013)
- PU Giacomoni, T Mammone and M Teri, Gender-linked differences in human skin, J Dermatol Sci 55(3) 144-9 (2009)
- M Baspeyras et al, Clinical and biometrological efficacy of a hyaluronic acid-based mesotherapy product: A randomized controlled study, Arch Dermatol Res (2013)
- M Kerscher, J Bayrhammer and T Reuther, Rejuvenating influence of a stabilized hyaluronic acid-based gel of nonanimal origin on facial skin aging, Dermatol Surg 34(5) 720-6 (2008)
- T Reuther, J Bayrhammer and M Kerscher, Effects of a three-session skin rejuvenation treatment using stabilized hyaluronic acid-based gel of non-animal origin on skin elasticity: A pilot study, Arch Dermatol Res 302(1) 37-45 (2010)
- SP Amin, RG Phelps and DJ Goldberg, Mesotherapy for facial skin rejuvenation: A clinical, histologic, and electron microscopic evaluation, Dermatol Surg 32(12) 1467-72 (2006)
- HS Yoon, SR Lee and JH Chung, Long-term topical oestrogen treatment of sun-exposed facial skin in post-menopausal women does not improve facial wrinkles or skin elasticity, but induces matrix metalloproteinase-1 expression, Acta Derm Venereol (2013)
- www.plasticsurgery.org/cosmetic-procedures/dermal-fillers-collagen.html (Accessed Jan 7, 2014)
- U Wollina, Facial rejuvenation for middle-aged women: A combined approach with minimally invasive procedures, Clinical Interventions in Aging 293 (2010)
- KR Beer, Combined treatment for skin rejuvenation and soft-tissue augmentation of the aging face, J Drugs Dermatol 10(2) 125-32 (2011)
- F Braccini and DM Dohan Ehrenfest, Advantages of combined therapies in cosmetic medicine for the treatment of face aging: Botulinum toxin, fillers and mesotherapy, Rev Laryngol Otol Rhinol (Bord) 131(2) 89-95 (2010)





!['We believe [Byome Derma] will redefine how products are tested, recommended and marketed, moving the industry away from intuition or influence, toward evidence-based personalization.' Pictured: Byome Labs Team](https://img.cosmeticsandtoiletries.com/mindful/allured/workspaces/default/uploads/2025/08/byome-labs-group-photo.AKivj2669s.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&fp-x=0.49&fp-y=0.5&fp-z=1&h=191&q=70&w=340)




