
Recently, an increased number of methods for determining the ultraviolet (UV) protection factor have been proposed. In fact, the past five years have seen the validation of three major methods by the ISO to evaluate sun protection, including in vivo SPF (ISO 24444), in vivo persistent pigment darkening (PPD, ISO 24442) and in vitro PPD (ISO 24443, to be published in 2012).1 Nevertheless, despite great effort, some methods based on photostability percentage are still not validated, and while several methods are published, none is generally used.2–5 This is mainly due to their lack of inter-laboratory reproducibility or their use of analytical chemistry techniques such as HPLC, which are excessively burdensome to implement.3 Clearly there is compelling interest in validating a method to determine the percentage of photo-protection that remains in a product after UV exposure, i.e., the photostability percentage.
Sunscreen products are intended to protect the wearer from harmful UV rays. In some cases, the molecules of UV filters that provide this protection by absorbing energy are fragmented. Consequently, the filters no longer play their role of absorbing UV radiation, thereby affecting the protective efficacy of the sunscreen.6–8 The degree of photostability is difficult to predict; some UV filters are photostable while others are unstable—not to mention that certain mixtures of filters can even be used to enhance the photostability of unstable filters.9, 10 It is therefore necessary to determine the photostability of UV filter combinations to develop efficient sunscreen products.
In previous studies,11, 12 the authors demonstrated that the absorbance value of a sunscreen depends on its being spread uniformly on the test substrate. This is one reason that spectroscopic methods to measure the absorbance of a thin layer of sunscreen spread onto an irregular substrate leads to issues of reproducibility. Here, the authors describe a new method for determining the photostability of sunscreen products based in part on the in vitro determination of the UVA protection factor as proposed by Colipa in 2011 for the irradiation aspect, and on the spectroscopy of a sunscreen in dilute solution for the absorbance measurement.
Materials and Methods
All spectroscopic data collected was based on transmission measurements of sun care products before and after irradiation. A long-arc xenon solar simulatora filtered with its original UV short cut-off filter (Ref: 56052371) and infrared (IR) dichroic mirror (Ref: 56052059) was used, which results in a spectrum extending over the UV and visible regions. The spectral irradiance at the exposure plane of this source is similar to the irradiance at ground level under a standard zenith sun, as defined by Colipa13 or in DIN 67501.14 This UV irradiance is within the following acceptance limits (measured at sample distance): 5–14 mW/cm2.
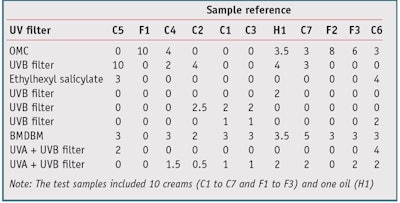
Protocol 1: This study used the high roughness, molded polymethylmethacrylate (PMMA) platesb recommended in the UVA Colipa method.1 The optimal amount of sunscreen per surface unit on these high roughness plates is 1.3 mg/cm².12, 15 Regarding the test procedure, 28.7 mg of each product (see Table 1) was dispensed and spread carefully over the PMMA plate. The samples were allowed to settle for 15 min in the dark at room temperature to ensure leveling of the products. A total of nine UV transmission spectra— i.e., from 290 to 400 nm, in 1-nm increments—were recorded at different locations on each plate. A transmittance analyzerc was then used to determine the diffuse transmission spectrum of UV radiation through the substrate before and after irradiation, followed by the photostability percentage.
Protocol 2: In this study, the in vitro UVA Colipa method also was used to test the same 11 products but the photostability percentage was calculated from the absorbance obtained by dilution spectroscopy. For this approach, extractions of the PMMA plate are prepared from both irradiated and non-irradiated samples, which are obtained as follows. First, the PMMA plate is placed in a 600-mL beaker with the rough surface facing upward and completely immersed with 45 mL of isopropanol. The beaker is covered with silver foil and placed in an ultrasonic bath for 5 min, then cooled with a water bath. This procedure is repeated three times before the plate is removed and rinsed with solvent. The solution obtained is poured into a 50-mL volumetric flask and the beaker is rinsed with 2 mL of isopropanol, which is also added to the flask. The flask is then closed and allowed to settle for 5 min, adjusted to 50 mL with isopropanol, closed and mixed. The extracted solutions are then placed in 1-mm quartz cellsd for transmittance analysisc.
Calculating Photostability
A product is photostable when its active UV filters show no significant degradation after irradiation. Thus, to quantify the photostability of sunscreen products, it is necessary to compare the degree of protection before and after irradiation to evaluate the degradation that occurred. Two percentages of photostability were therefore calculated (see Figure 1, Figure 2, Figure 3 and Figure 4) using both protocols for the 11 test products, one in the UVB range (SPF) and the other in the UVA range (PPD), to get an overall idea of the quality of solar products studied. However, other calculations are also possible, i.e., with other irradiance spectrums or without any action spectrum.
As noted, for protocol 1, calculations were made per the Colipa method for the in vitro determination of UVA protection factor based on the results of the thin film absorbance measurements of products on PMMA plates. The irradiation time was calculated from the matrix provided by Colipa and is proportional to the value of the UVA protection before irradiation.1 Since the in vitro SPF has not been adjusted to in vivo values, the value of c will be equal to 1 for all measurements. For protocol 2, the same method proposed by Colipa was used for the irradiation step, where c = 1, but the percentage of photostability was calculated from the absorbance obtained by dilution spectroscopy.
For protocol 2, the percentage of extraction must first be verified to ensure that enough product was extracted from the PMMA plate. This percentage corresponds to the quantity of product that could be extracted from the plate compared with the quantity that was originally dispensed. For this, the absorbance of the diluted product is compared directly with the absorbance of the solution of the product extracted from a non-irradiated plate. This calculation is based on the additivity absorbances for a product in solution per the Beer-Lambert Law (see Figure 5). The absorbance taken into account in this calculation is the absorbance corresponding to a mass of 28.7 mg and is derived by the equation shown in Figure 6.
Results and Discussion
As noted, the importance of both spreading the test sunscreen formulations evenly onto the substrate and the surface characteristics of the substrate itself were previously demonstrated.11, 12 These are key to performing transmission spectroscopy on a thin film. Until this is mastered, it is not possible to obtain reproducible intra- or interlaboratory results. This methodology, which proved difficult, was used first to calculate the photostability percentage.
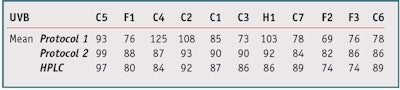
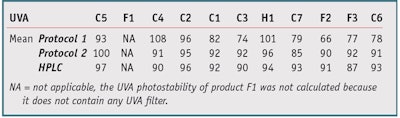
Protocol 1: The thin film absorbance measurements on PMMA substrates were made twice, once before and once after irradiation, before determining the ratio; irradiation was calculated from the initial UVA absorbance. The photostability percentage was calculated using protocol 1 for the 11 products shown in Table 1. These initial results, shown in Table 2, yielded the following insights. Products C4, C2 and H1 showed a photostability percentage above 100%, as the SPF (or PPD) after irradiation was higher than the SPF (or PPD) before irradiation. In addition, Products C1 and C3 had the same filter combinations but different photostability percentage. And some products showed good reproducibility— e.g., C5, F1, C4, C3, F2 and C6, while others were clearly inadequate—e.g., C2, H1, C7 and F3.
These results can be explained by the fact that the thin sunscreen films rearrange themselves on the PMMA substrate during irradiation, which would result in an artificial increase or decrease of the photostability percentage, or variable results from one analysis to the other (see Rearrangement). So these results are not always directly related to the “real” degradation of the product. Add to this the problems of intra-laboratory reproducibility and the photostability percentage above 100% and it becomes clear that this protocol cannot be used as it currently is.
While protocol 1 is based on the method for determining UVA protection as recommended by Colipa (PPD in vitro),1 it does not seem suitable for the determination of photostability due to its calculations; i.e., c = 1, and the photostability percentage being a ratio of absorbance before and after irradiation— when there is great potential for variances in these values. Conversely, for the in vitro PPD method, only the values after irradiation are used and the in vitro value is adjusted to the in vivo value thanks to c; so even if these in vitro PPD values are subject to some variability, this method is acceptable, as has been demonstrated by international ring tests [ISO norm, to be published in 2012].16
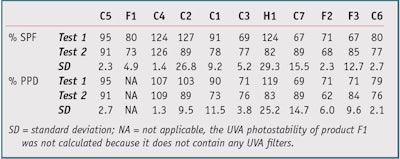
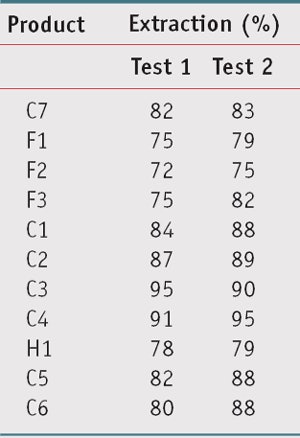
Protocol 2: Thus, protocol 2 was set up, which initially is identical to protocol 1, but once the plates are irradiated, as described previously, the product is extracted from the substrate with a solution of ethanol, following which the solution is analyzed by a spectrophotometer. The absorbance before irradiation—and after calculation, the SPF and PPD—was compared with the absorbance after irradiation (SPF and PPD) to determine the photostability percentage, as for protocol 1. The extraction percentages were first calculated to verify that they were all sufficiently high and relatively homogeneous according to the formulations (see Table 3); all, except for F2, were well above 75%. Following protocol 2, the photostability percentage of the 11 test products was again calculated.
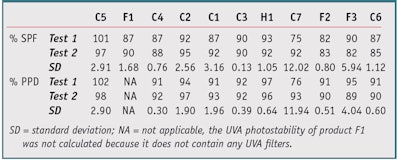
Table 4 shows the improvement brought about by this new protocol on the calculations of photostability percentage in both the UVA and UVB range. Except for Product C5 (101% on test 1), no product exceeded 100%. Products C1 and C3, comprising an equal mix of filters, had equivalent photostability percentages. All results showed good reproducibility with a standard deviation of less than 5, except for C7 (SD = 12.02) and F3 (SD = 5.94), with a clear improvement over protocol 1.
Further, photostability percentage does not seem to be directly related to the extraction percentage; while Products C6 and F3 had the greatest difference in extraction percentage, they had a similar photostability percentage, as shown in Table 5. Protocol 2 solves the reproducibility and inconsistency issues of protocol 1 because the ratio of absorbance in dilute solutions is not subject to the problems associated with thin film distribution on the PMMA plates. A second trial confirmed this analysis.
The in vitro PPD test proposed by Colipa requires that multiple reflections during UV exposure be avoided. This can be controlled by placing a matte, dark, nonreflective material behind the PMMA substrate to minimize the scattering of radiation back through the plate. The influence of a black sheet under the PMMA plate was randomly tested on samples H1 and F3 using the two protocols (see Figure 7).
With protocol 1, the photostability percentages varied significantly, depending on whether the black sheet was used or not. In the UVB range, Product H1 varied between 123% and 81%, and product F3 varied from 66% to 85%. With protocol 2, however, the photostability percentage was consistent. Product H1 varied from 95% to 92% and product F3 varied from 90% to 82%. Also, removing the black sheet did not alter the photostability results obtained by dilution. Since the sun products are diluted in a solvent, the main effect influencing the thin film spectroscopy—i.e., the distribution of the product on the substrate—has no effect. The product is evenly distributed in the solution, so there is no significant difference between the results despite the change in condition.
This protocol keeps the effect of the thin film during the irradiation step, which is similar to in vivo tests, but eliminates the problems of reproducibility of the transmission measurements. Calculation of the photostability percentage is relative—i.e., the ratio of absorbance before and after irradiation, and thus it is not affected by the dissolution of the filters. This is in contrast to calculations based on absolute values such as the UVA in vitro protection factor (PPD), which must be made on a thin layer in order to take into account the effect of the vehicle and thus be correlated with in vivo values. Also, the photostability percentage of the different products may seem high since they are all above 80%. However, note that the in vivo SPFs are low to high, which means their PPD or UVA protection would only be between 3 and 10, and the products receive an irradiation dose proportional to their UVA protection, which accounts for the low degradation.
HPLC and Protocol 2
At the end of 2003, Colipa proposed a method for determining the in vitro photostability of UV filters in cosmetic products based on an HPLC method.3 So to determine whether a link exists between the Colipa HPLC method and protocol 2, HPLC analyses of the solutions were also performed before and after irradiation.
HPLC analysis revealed the percentage of each UV filter used in the solutions, making it relatively easy to determine the absorption spectrum between 290 nm and 400 nm of each solution, and thus the photostability percentage in the UVB and UVA range. This percentage was compared with the percentage obtained through the previously described spectrophotometric measurements of protocols 1 and 2. If the thin film affected the photostability results, a difference should be seen between the results obtained with protocol 1 on PMMA plates but not with those obtained by protocol 2, in solution.
As Table 6, Table 7, Figure 8 and Figure 9 clearly show, with some products (C4, C2, C3, H1, C7 and C6), HPLC method and protocol 1 gave very different results (> 10% difference). Conversely, the results obtained by HPLC and protocol 2 were similar for 10 products, whether in the UVB or UVA range. Only Product F3 gave results significantly different from the HPLC results. Its photostability percentage was 86%, a very high value for this combination of filters, so tests should be repeated on this product. This could again be explained by rearrangement; in this study, Products C4, C2, C3, H1, C7 and C6 have galenic properties that allow for their rearrangement on the media during irradiation, which would modify the properties of absorbance of the product and thus disturb the calculation of the photostability percentage with protocol 1.
There are also many products whose film does not change upon irradiation; C5, F1, C1, F2 and F3, in this study. The photostability percentage in this case would be equal regardless of the method used, i.e., protocol 1, protocol 2 or HPLC. These results also confirm the relatively high photostability percentage found with protocol 2, demonstrating that even with relatively photo-unstable mixtures, the products retain a degree of photostability if the protection factor is low to high. Thus, with protocol 2, photostability percentage values can be obtained that are not biased by the possible rearrangements of the thin film since the product is dissolved in a solvent. These results give the real degradation percentages afflicting the products, as confirmed by HPLC, but without the constraints of analytical techniques.
Conclusion
For many years, UV transmission spectroscopy of thin films has faced problems of reproducibility, which are mainly due to issues of controlling the spreading of the product on the substrate. Further, a method for determining the photostability percentage based solely on this technique could become increasingly inaccurate since it involves taking the absorbance measurement twice in the calculations—once before and once after irradiation. The resulting ratio will therefore result in significant variations. Tests on 11 products, described here, confirm this assumption.
The protocol was thus changed to involve a spectroscopic technique in dilute solution in order to avoid the absorbance measurements of thin films. The photostability percentages obtained in the UVB and UVA regions for the same 11 products were then found to be reproducible and in line with the results obtained by HPLC. This method therefore provides the real photostability percentage of the product, not modified by the redistribution of the thin film, and seems to be a good candidate for validation in a ring test.
References
- Method for in vitro determination of UVA protection, Colipa website, available at www.colipa.eu/publications-colipa-theeuropean- cosmetic-cosmetics-association/ guidelines.html?view=item&id=33 (Accessed Jan 11, 2012)
- L Ferrero, M Pissavini, S Marguerie and L Zastrow, Photochemical behavior assessment of sunscreen preparations by in vitro UV spectroscopy, Intl J Cos Sci 26, 318 (2004)
- Colipa method for the in vitro determination of photostability of UV filters in cosmetic products, Final draft version, Guideline/protocol (2003)
- B Herzog and K Sommer, Investigations on photostability of UV absorbers for cosmetic sunscreens, XXI IFSCC International Congress proceedings, Berlin (2000 Berlin)
- LR Gaspar and PMBG Maia Campos, Evaluation of the photostability of different UV filter combinations in a sunscreen, Intl J Pharmaceutics 307,123–128 (2006)
- S Schalka and V M Silva dos Reis, Sun protection factor: Meaning and controversies, An Bras Dermatol 86(3) 507–515 (2011)
- C Bonda, Research pathways to photostable sunscreens, Cosm & Toil 123 (2) 49–60 (2008)
- E Chatelain and B Gabard, Photostabilization of butyl metoxydibenzoylmethane (avobezone) and ethylhexyl methoxycinnamate by bis-ethylhexylphenol methoxyphenyl triazine (Tinosorb S), A new UV broadband filter, Photochemistry and photobiology 74(3) 401–406 (2001)
- L Ferrero, M Pissavini, S Marguerie and L Zastrow, In vitro spectroscopy: How to assess the photostability of sunscreen preparations by measuring changes in specific UV indices, poster at XXII IFSCC International Congress Edinburgh (2002)
- B Herzog, M Werhle and K Quass, Photostability of UV absorber system in sunscreens, Photochem and Photobiol 8 869–878 (2009)
- L Ferrero, M Pissavini, A Dehais, S Marguerie and L Zastrow, Importance of substrate roughness for in vitro sun protection assessment, IFSCC magazine 9(2) (Apr/Jun 2006)
- L Ferrero, M Pissavini and O Doucet, How a calculated model of sunscreen film geometry can explain in vitro and in vivo SPF variation, Photochem Photobiol Sci 9 540–551 (2010)
- Colipa SPF test method, Ref 94/289, available at https://single-market-economy.ec.europa.eu/sectors/cosmetics_en (Accessed Jan 17, 2012)
- Deutsches Institut für Normung (DIN): Experimental evaluation of erythema protection of external sunscreen products for the human skin, revision of DIN 67501 (Sep 1999)
- M Pissavini, S Marguerie, A Dehais, L Ferrero and L Zastrow, Characterizing roughness: A new substrate to measure SPF, Cosm & Toil 124(9) 56–64 (2009)
- PJ Matts et al, The Colipa in vitro UVA method: A standard and reproducible measure of sunscreen UVA protection, Intl J of Cos Sci 32 35–46 (2010)








!['We believe [Byome Derma] will redefine how products are tested, recommended and marketed, moving the industry away from intuition or influence, toward evidence-based personalization.' Pictured: Byome Labs Team](https://img.cosmeticsandtoiletries.com/mindful/allured/workspaces/default/uploads/2025/08/byome-labs-group-photo.AKivj2669s.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&fp-x=0.49&fp-y=0.5&fp-z=1&h=191&q=70&w=340)


