*A portion of this work was presented at the 28th IFSCC Congress, Paris (2014)
In ayurveda, the Terminalia chebula (TC, INCI: Terminalia Chebula Fruit Extract) herb, and especially its fruits, are well-known. It is one of the key ingredients used in triphala or “three fruits”—the most frequently prescribed herbal remedy in ayurveda.
Among Tibetans, TC is highly revered for its purifying attributes; in fact, its fruit is depicted in the hands of the medicine Buddha in sacred paintings. These small, round and brownish-colored fruits were historically used to normalize the general balance of the body.
Two reviews of TC’s pharmaceutical and biochemical effects recently were published.1, 2 In relation, this paper describes several tests to examine the skin benefits of a hydrolyzable, tannin-enriched Terminalia chebula fruit extract. The biological activities previously reported for water-soluble TC extracts appear to be due to the presence of hydrolyzable tannins; more specifically chebulinic (see Figure 1) and chebulagic acids (see Figure 2). Commercially available extracts of TC typically contain ~33% of total hydrolyzable tannins; however, their content may vary as low as 20%.2
The TC extract used for the described studies was therefore standardized for total hydrolyzable tannins (> 50%) as well as chebulinic and chebulagic acids. The results indicate that a defined, water-soluble TC fruit extract can be used topically for preventive and restorative anti-aging and skin tone-evening effects.
Preventive Anti-aging Tests
TC was extracted from the edible fruits of T. chebula using water, then spray-dried. As noted, the extract was standardizeda for total hydrolyzable tannins ≥ 50%—typically ranging from ~55% to ~70%; also standardized were chebulinic acid ≥ 20% and chebulagic acid ≥ 15%. Free gallic acid content in the commercial batch was maintained at < 5% and the statistical significance of all results was calculated at p ≤ 0.05 using paired t-tests.
Quenching properties: Radical and non-radical quenching properties of the extract were measured (in µmole Trolox equivalent/g) using a well-established method.3
Xanthine oxidase (XO) inhibition: A solution of the test material was prepared (20 mg/mL) and diluted in type 1 sterile water immediately before the experiment. The effects of the test material on XO-mediated free radical (superoxide) generation were assessed according to Palanisamy et al.4
This approach uses a hypoxanthine/XO/tetrazolium salt (NBT) system, whose reaction product (formazan) is quantified at 570 nm by spectrophotometerb. Allopurinol (AP) serves as the positive control and double-distilled water as the negative control.
Matrix metalloprotease-1 (MMP-1) inhibition: The test material was dissolved and diluted in type 1 sterile water (20 mg/mL) immediately before the experiment, then incubated at different dilutions with pure human MMP-1 enzymec. For the negative control, sterile water was used; for the positive control, N-isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic acid (NNGH), a broad-spectrum MMP inhibitor was used.
The proteolytic activity of MMP-1 was measured by fluorimeterd at excitation/emission wavelengths of 485 nm/530 nm using quenched fluorescent gelatine as the substrate. The fluorimeter was standardized to the water control.
MMP-2 inhibition: For MMP-2 measurements, the procedure was the same as for MMP-1 except the material was incubated with pure human MMP-2 enzymef. Quantification was performed using a chromophoric reaction product, i.e., DTNB or Ellman’s Reagent, at 405 nm and measured by spectrophotometerb, which was standardized to the water control.
MMP-3 inhibition: MMP-3 assessment used the same procedure as for MMP-1, except the material was incubated with pure human MMP-3 enzymeg. Quantification was carried out in the same manner as for MMP-2.
Lipooxygenase (LOX) inhibition: To test for LOX inhibition, the TC extract was dissolved (10 mg/mL) and diluted in type 1 sterile water immediately before the experiment. Samples were tested at different dilutions using type V LOX from soybeans, with linoleic acid as the substrate. The generation of hydroperoxide reaction products was followed at 234 nm with a diode array spectrophotometerh and processed using software, as described in the literature.5
Cyclooxygenase-2 (COX-2) modulation: To assess COX-2 gene modulation, the TC ingredient was dissolved and diluted in type 1 sterile water (20 mg/mL) immediately before testing. Human chondrocytesj were placed in 12-well plates at 100,000 cells/well in growth mediumk and exposed to the test ingredient the next day. The cells were then deprived of serum and treated with 100 mg/mL TNF-α, with and without the test material.
After 24 hr, cells were lysed and total RNA extracted with an RNA spin kitm. The purity and quantity of each RNA preparation was validated and gene expressions were measured by real-time quantitative PCRn. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) “housekeeping” genes, and genomic contamination control (INTR2) primers were usedp as well as other primersq. Changes in expression were standardized to the expression of the GAPDH housekeeping control gene.
Anti-glycation tests: To assess anti-glycation properties, the TC ingredient was dissolved in dimethyl sulfoxide (DMSO) (20 mg/mL) and diluted with type 1 sterile water immediately before testing at 10 µg/mL, 50 µg/mL and 100 µg/mL. Aminoguanidine (74 µg/mL) served as the benchmark. Equivalent concentrations of DMSO (0.5%, 0.25% and 0.05%) were used as the negative controls.
All samples were incubated for 10 days at 37°C in the presence or absence of 500 mM glucose. The incubations additionally contained 10 mg/mL of serum albumin and 0.025% sodium azide, an antimicrobial agent.
Non-enzymatic glycation measurements were taken based on the method described by Suzuki et al.,6 with minor modifications. Briefly, the test material was added to 96 black well plates containing 10 mg/mL albumin in the absence or presence of 0.5 M glucose and the plates were sealed. On day one, initial non-tryptophan fluorescence was assessed by fluorimeterd at 409 nm/460 nm. After 10 days of incubation at 37°C, fluorescence was read again and the signal increase between the first and the last day of the incubation was calculated for each experimental point.
Finally, the difference between the signal increase for each sample incubated in the presence and absence of glucose was obtained and standardized to the water control.
Restorative Anti-aging Tests
Collagen stimulation: To test the effects of TC extract on collagens I and III, the ingredient was dissolved (20 mg/mL) and diluted in type 1 sterile water at different concentrations immediately before the experiment. Normal human dermal fibroblastsr from the facial skin of a 42-year-old female donor were grown in medium for propagation, then in a more defined DMEM with 5% serum for the assay.
Cells were plated at 5,000 cells/well in a 96-well plate and exposed to the test materials for three or five days for type I and III collagen quantification, respectively. Cell-culture conditioned media was harvested and tested for types I and III collagen by sandwich enzyme-linked immunosorbent assay (ELISA) using affinity-purified antibodies, followed by streptavidin-avidin-HRP conjugate and 2,2ʹ-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)(ABTS), according to standard ELISA protocol.7, 8
Hyaluronidase inhibition: To determine its effects on hyaluronidase inhibition, the TC ingredient was dissolved in H2O (20 mg/mL) immediately before the experiment. Further dilutions were made in sterile water and test materials were incubated at different concentrations with pure human MMP-1 enzymec.
Sterile water served as the negative control, while the positive control was tannic acid, as described by Sumatran et al.9 The effect of the test materials on the activity of hyaluronidase was determined by precipitating the non-digested hyaluronate with 5% cetylpyridinium chloride and measuring the related turbidity at 595 nm with a spectrophotometerb.
Glycation reversal, method one: Another test examined the ability of TC to revert the non-enzymatic glycation (NEG) of proteins in the albumin/glucose/extracellular matrix (ECM) ELISA model system. This methodology was based on that by Lee et al.,10 with modifications.
Briefly, glycated serum albumin was prepared by the incubation of bovine serum albumin (BSA, 10 mg/mL) with glucose (0.5 M) for 28 days in the presence of sodium azide (250 µg/mL) at 37°C, under an adhesive seal. BSA incubated with phosphate-buffered saline (PBS) instead of glucose also was prepared and served as the non-glycated negative control.
Samples of glycated albumin along with the non-glycated negative control (100 µL/well) were then transferred to a 96-well plate coated with collagen-based cell culture ECMs and incubated for six days to allow cross-linking to form between proteins. On day six, test materials were added and samples were incubated for an additional three days. 2-Amino-4,5 dimethylthiazole hydrobromide (2A4,5DMT)t was used as the positive control.
The experiment was terminated by washing wells with PBS-T detergent (0.1%) and detecting ECM-linked BSA by direct ELISA, with anti-albumin primary goat antibody (2 µg/mL in PBS) followed with a fluorescein isothiocyanate (FITC) linked secondary (anti-goat) antibodyu. The fluorescent signal was quantified by fluorimeterb at 485 nm/530 nm.
Glycation reversal, method two: To validate the reproducibility of method one results, the same experiments were repeated using a different secondary antibody with a corresponding readout system. Instead of FITC-linked secondary antibody and fluorescent readout, the test used a biotinylated secondary (anti-goat) antibody, streptavidin-avidin-HRP conjugate, followed by colorimetric readout at 405 nm with ABTSv using a microplate spectrophotometerw and softwarex. Statistically significant variations (p < 0.05) were defined as ≥ 15% from either the glycated or non-glycated BSA control, as calculated by double-tailed t-test.
Tyrosinase inhibition: To assess effects on tyrosinase, the TC extract was dissolved (20 mg/mL) and diluted at different concentrations with type 1 sterile water immediately before the experiment. Tyrosinase activity was measured following modified methods of Pomerantz11 and Ochguchi et al.12
Mushroom tyrosinase stock solution was prepared in PBS at 2000 U/mL and stored at -20°C in 1 mL aliquots. The final concentration was 5 U/well (25 U/L). The L-DOPA concentration was 5 mM. The tyrosinase substrate was prepared in PBS immediately before the assay. The reaction was initiated by adding tyrosinase. Assays were performed in 96-well flat-bottom microtiter plates and read at 450 nm by microplate readerb.
Peroxidase inhibition: Lastly, to test peroxidase inhibition, the TC material was dissolved at 20 mg/mL and diluted to different concentrations with type 1 sterile water immediately before the experiment.
Peroxidase activity was measured using horseradish enzyme and its chromogenic substrate 2, 2ʹ-azinobis (3-ethylbenzothiazoline 6-sulfate) (ABTA) at 405 nm in the microplate readerb. Identical experimental conditions without peroxidase were used for background subtraction.
Clinical Studies
Test creams and expert grading: In a blind clinical study, 17 healthy, but photo-aged female subjects, ages 40-65, were recruited and provided informed consent. All subjects used only simple soap and abstained from moisturizers for at least one week prior to treatment conditions. All test products were supplied in identically packaged, coded containers.
Subjects applied the test cream (see Formula 1) twice daily, morning and evening, to their entire face for three months. Results were compared with baseline measurements.
Clinical and subjective assessments of subjects’ facial skin were made at baseline and following four, eight and 12 weeks of product use. The following parameters were assessed:
- fine lines/wrinkles
- roughness and dryness
- skin tone
- skin elasticity and firmness
- radiance
- brightening
- overall eye area appearance
The assessment for each parameter was performed at baseline using the following five point ordinal severity scale: 0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe.
Silicone profilometry: At each clinical visit, a single silicone replica was made of the target area on each subject’s face and a photographic record was kept for subsequent relocation. Comparative analysis of skin profilometry was conducted using surface roughness and wrinkle depth analysis.
The heights of the replicated wrinkles were measured using a profilometer; Ry (depth) and Ra (mean roughness) values were recorded with each measurement. The area scanned from each sample was clearly mapped so as to determine the same area at weeks four, eight and 12.
High resolution images: At each time point, a series of high resolution digital photographs also was collected using a photo booth equipped with a 10-megapixel digital camera with a 6× zoom. Subject positioning was reproduced upon return visits.
A light booth was used to provide controlled and reproducible lighting conditions. This consists of an array of eight, equally spaced fluorescent tubes in a semicircular configuration. This software-driven system allows the position and expression of test subjects to be aligned to a highly accurate degree.
Results and Discussion: Preventive Anti-aging
Slowing the aging process can be achieved by antioxidant protection to limit direct oxidative damage to the cells, proteins and DNA. In addition, controlling inflammation to minimize skin damage, inhibiting MMP activity to protect extracellular proteins, and reducing glycation to maintain elasticity and decrease the yellowing of skin tone and changes in the dermis all can reduce the effects of aging. According to the results, described next, the TC extract appears to satisfy these criteria.
Quenching properties: Oxidative stress occurs when the balance between oxidation and anti-oxidation is tilted in favor of the former. Besides radicals, oxidase enzymes are equally responsible for generating oxidative stress, specifically superoxide anion.
The antioxidant activity of TC extract (non-standardized)13, 14 and some isolated compounds of TC was reported earlier.15 The present study further confirmed that standardized TC acts as a broad-spectrum antioxidant (see Table 1) and is capable of quenching all major radicals and non-radicals generated in the skin upon UV exposure.
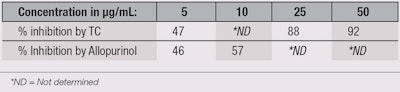
XO inhibition: TC also provides dose-dependent inhibition against XO. At 5 µg/mL, TC and allopurinol (positive control) showed comparable activity against XO. The ED50 of both TC and allopurinol was 6 µg/mL; results are summarized in Table 2. However, at the dose tested, TC did not show statistically significant inhibition against other pro-oxidant enzymes, such as NADPH oxidase, glucose oxidase and myeloperoxidase.
XO catalyzes the production of uric acid, nitric oxide and reactive oxygen species (ROS). These entities can damage DNA, RNA and proteins; inactivate enzymes; oxidize amino acids; and convert poly-unsaturated fatty acids to lipids.16, 17 TC could therefore have clinical relevance against such damage, given its inhibition of XO.
LOX inhibition: Skin aging and inflammation are critically linked. The inflammatory response is a double-edged sword; when properly orchestrated, it results in the clearing of foreign molecules and invading pathogens from the body. Uncontrolled, it may lead to organ damage, sepsis and even cancer. Many pathological manifestations of the inflammatory response are mediated by pro-inflammatory enzymes, cytokines and other inducible gene products.
As noted, TC was tested at two different concentrations against type V LOX from soybeans, with linoleic acid as a substrate. The ingredient was found to exhibit complete inhibitory activity against LOX at 100 µg/mL (data not shown).
COX-2 modulation: Contrary to the literature, which reports that chebulagic acid, one of the major tannins of TC, has strong COX-2 inhibitory activity,18 the TC extract was not found to inhibit COX activity. On the other hand, at 5 µg/mL, the TC extract significantly down-regulated both COX-1 (-70%) and COX-2 (-80%) genes; at this level, though, it exhibited no statistically significant down-regulation of the LOX gene (data not shown).
MMP inhibition: A major characteristic of aged and pre-maturely aged skin is the high degree of fragmentation in the dermal collagen matrix.19 MMPs play a major role in degrading collagen, in turn affecting the structural integrity of the dermis. Normal skin production is balanced by natural tissue inhibitors of metalloproteinases (TIMPs); however, UV light is reported to enhance the synthesis of MMPs, leading to further collagen destruction. This imbalance advances the aging process.
TC was found to significantly inhibit the activities of MMP-1, MMP-2 and MMP-3 (see Figure 3). Thus, it would be expected to provide strong ECM protection in vivo, which in turn would balance collagen production and degradation.
Glycation: Glycation is a slow, nonenzymatic reaction between free amino groups in proteins and sugar such as glucose or ribose. An advance glycation end-product (AGE) is the result of a chain of chemical reactions after an initial glycation reaction.
AGEs affect almost every type of cell and molecule in the body and are thought to be one of the key factors in skin aging. They stiffen and weaken the collagen and elastin in skin. When this happens, the elasticity, plumpness and youthfulness of skin deteriorate and fine lines start to appear.
Using skin equivalents, Pageon et al. demonstrated several changes in skin after collagen glycation.20 For example, UV exposure on AGEs generated ROS in the ECM.21 Besides photo-oxidative damage, increased cellular carbonyl stress mediated by glycation can also cause skin deterioration.22 AGEs further induce NF-kB activation and cytokine expression.23
The TC ingredient had a strong, dose-dependent anti-glycation effect (see Figure 4) and was found approximately seven times more effective than aminoguanidine, the gold standard.
In relation, one of the key hallmarks of glycation is the yellowing of skin, often seen prematurely in smokers. Thus, it would be expected that a formula containing TC would reduce the yellowing of skin.
Results and Discussion: Restorative Anti-aging
Collagen stimulation: Naturally aged and photoaged skin share important molecular features including connective tissue damage, elevated MMP levels, reduced levels of collagen production, skin moisture loss, and uneven pigmentation.
Collagen is centrally involved in the formation, distribution and function of fibrillar and microfibrilliar networks in different tissues. Collagen I, in particular, is a major structural protein in human skin and, by mass, the most abundant protein in the human body.
The association of age-dependent collagen loss with the thinning and increased fragility of elderly skin has long been appreciated. TC, however, was found to stimulate both collagens I and III (see Figure 5), thereby providing ECM structural integrity.
Hyaluronidase inhibition: The most dramatic histochemical change observed in senescent skin is the marked disappearance of epidermal hyaluronic acid (HA). The degradation of HA in the ECM decreases viscoelasticity, leading to a loss in structural integrity. Reports also have confirmed that chronic, repetitive UVB irradiation induces the loss of HA from murine dermis.24
In relation, in recent years, hyaluronidases (HAases) have received much attention due to their ability to abruptly alter HA homeostasis,25 preferentially degrading HA and producing unsaturated disaccharides or tetrasaccharides.
These breakdown products have dual effects. First, they generate a wide range bioactive oligosaccharides having angiogenic, pro-inflammatory and immunostimulatory properties. This impairs the capacity of the ECM to hold metal ions, growth factors, cytokines and various enzymes for signal transduction in reserve. HAase inhibitors are thus potent regulators, helping to maintain the balance between the anabolism and catabolism of HA.26
TC showed dose-dependent and statistically significant HAase inhibitory action; 17% at 50 µg/mL and 44% at 100 µg/mL (see Figure 6). In relation, HAse inhibition (IC50 0.86 mg/mL) by the tannin 1, 2, 3, 4, 6-penta-O-galloyl-b-D-glucose recently was reported by Kim et al.27 Triphala guggulu, a blend of the three fruits Phyllanthus emblica, T. chebula and T. bellerica with Comniphora mukul oleoresin, also showed HAse inhibitory activity.28
Glycation reversal, method one: AGE cross-links are permanent and irreversible complexes, formed when glucose binds to target proteins including collagen and elastin. AGE accumulation in collagen is thought to affect changes in the elasticity, ionic charge, thickness and turnover of basement membrane components.29
One limitation of AGE inhibitors is they cannot affect pre-existing AGE cross-links. Therefore, another way to reduce levels of AGEs is to use a cross-link breaker. Alagebrium chloride and thiazolium chloride (ALT-711) are well-known AGE cross-link breakers, which have been investigated in hypertension trials (Phase II).30
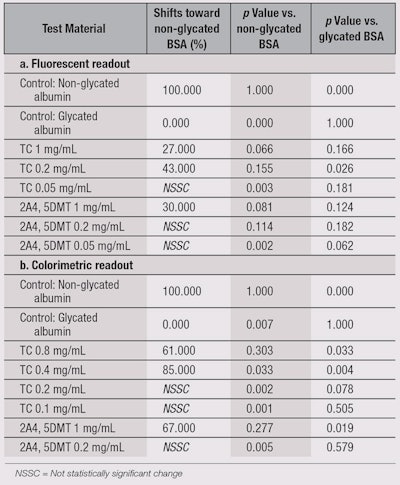
In relation, TC extract also was found to break collagen cross-links. As illustrated in Table 3a, a statistically significant difference was observed in the binding of anti-albumin antibody to non-glycated and glycated BSA; the recognition of glycated BSA by the anti-albumin antibody was much weaker than of the native protein.
Furthermore, when incubated with glycated BSA for three days, TC exhibited glycation-reversing activity, with the optimal effect recorded at 0.2 mg/mL. This breaking activity was approximately seven-fold stronger than the positive control at 1 mg/mL.
Glycation reversal, method two: To ensure the reproducibility of results, as noted previously, the same experiment was conducted using a different secondary antibody and readout system. The TC ingredient had strong activity at 0.4 mg/mL and 0.8 mg/mL, enabling a statistically significant shift in antibody recognition toward the level recorded with non-glycated BSA (see Table 3b).
It was concluded that TC at 0.4 mg/mL and 0.8 mg/mL partially reversed albumin glycation, causing a shift in its tridimensional structure back toward its native state; thus confirming the results described in Table 3a. This activity was comparable with the positive control 2A4, 5DMT, which exhibited glycation-breaking activity at 1 mg/mL, technically validating the results.
In relation, recently, Lee et al. found chebulic acid (IC50 = 1.46 ± 0.05 mM) to break collagen cross-links induced by glycol-BSA; this study showed chebulic acid had a 50-fold stronger breaking activity than ALT-711, a well-known cross-link breaker (IC50 = 72.2 ± 2.4 mM).31
Further research is required to determine whether TC has glycation-reversing activity toward other macromolecules—such as ribonucleic acids—and its mechanism of action. To the best of the authors’ current knowledge, however, this is the first report of glycation reversal from a commercially available cosmetic ingredient.
Tyrosinase and peroxidase inhibition: Among the array of human skin colors are also many pigmentary disorders, ranging from hypopigmentation to hyperpigmentation. Hyperpigmentary problems, including post-inflammatory hyperpigmentation, solar lentigos and melasma, occur widely in the human population and are thus of broad interest to control.
Key players regulating human skin pigmentation include melanocytes in the epidermis, which synthesize melanin, and the neighboring keratinocytes that receive and distribute it in upper layers of the skin. Extrinsic factors directly and indirectly affecting skin pigmentation include UV radiation.32
The enzymes regulating melanin synthesis are tyrosinase and peroxidase. They are involved in converting DOPA to DOPAchrome, and dihydroxyindole (DHI) and dihydroxyindole carboxylic acid (DHIC) to melanin (see Figure 7).
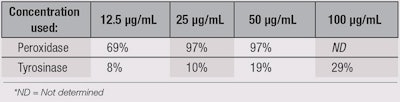
In relation, TC showed moderate tyrosinase inhibition, whereas it had strong peroxidase inhibition—nearly 100% at 25 µg/mL (p = 0) (see Table 4). This in vitro inhibitory action could have clinical relevance. In relation, several authors previously reported on the tyrosinase and melanin inhibitory activities of non-standardized TC extracts.33-35
To the best of the current authors’ knowledge, this article is the first to report on TC’s peroxidase inhibitory activity, which interestingly, was more pronounced than its tyrosinase inhibition (see Table 4).
Results and Discussion: Clinical Study
Finally, the TC extract was formulated into a finished skin care product (see Formula 1) and tested, as described previously, by twice daily application. Note that since the key biologically active components in TC are polyphenolics, they tend to develop color over time; thus, a small amount of propyl gallate can be added to reduce this coloration.
Although some improvements in most skin parameters were noted after just four weeks, significant improvements were noted after eight weeks. These improvements continued to increase, more rapidly, through 12 weeks of product application, resulting in significant improvement in lines and wrinkles, pigmentation, elasticity and firmness.
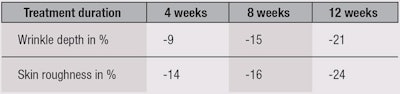
An overall reduction in photodamage also was observed, without any undesirable effects. Results of subjective parameters are summarized in Figure 8 and Figure 9, and reductions in wrinkle depth and skin roughness are summarized in Table 5.
Clinical grading and comparative analyses of skin profilometric measurements were performed at baseline and after four, eight and 12 weeks of product application. After eight weeks of application, significant reductions in wrinkle depth and roughness were observed versus the baseline (see Table 5).
These changes were even greater after 12 weeks of application. In relation, the wrinkle depth at eight weeks was significantly less than at four weeks, indicating a degree of cumulative benefits over time.
These significant improvements in fine lines and wrinkles, elasticity and firmness—as well as evenness of skin tone—provided validation of the in vitro results (see Figure 8 and Figure 9). Significant reductions in skin pigmentation were observed with use of the test product versus the baseline (see Figure 10, Figure 11 and Figure 12).
As noted, one of the hallmarks of glycation is the yellowing of skin tone, often seen prematurely in smokers. Interestingly, with use of the test product, a reduction in yellowish skin tone was observed in a few subjects (see Figure 11). A significant improvement in dark circles around the eyes also was observed in a some subjects (see Figure 12).
Conclusion
This paper examines the science behind skin benefits provided by TC extract to determine the mechanisms by which they are attained: including preventative and restorative anti-aging effects and skin pigmentation control.
The preventative anti-aging benefits of TC are believed to involve:
- broad-spectrum antioxidant protection, to limit direct oxidative damage;
- inflammation control, by inhibiting COX and LOX effects and minimizing inflammation-induced skin damage;
- the inhibition of MMP-I, II and III activities, to protect ECM proteins; and
- the reduction of glycation, to maintain elasticity and changes in the dermis associated with the aging process.
Restorative anti-aging effects include the:
- stimulation of collagen I and III;
- inhibition of hyaluronidase activity, thereby maintaining hyaluronic acid homeostasis; and
- breakage of glycation cross-links, thereby reversing some loss of elasticity, thickness and turnover of basement membrane components.29
Skin pigmentation control is believed to involve both tyrosinase and peroxidase enzyme inhibitory activities, as well as the breakage of AGE cross-links.
The results of standardizing the extract also demonstrate that tannins present in the TC are the active ingredients—not gallic acid, as portrayed by previous studies.36, 37 Gallic acid is an artifact obtained from the hydrolysis of tannins during processing of the extract.
The 12-week pilot clinical study further confirms the bioactivity of TC in vivo, which reduced fine lines and wrinkles as well as pigmentation. Recently, another clinical study demonstrated AGEs are involved in both intrinsic and extrinsic aging processes, and that their accumulation increases with age and is amplified by sun exposure.38, 39
Additionally, improvements in reducing a yellowish skin tone were noticed in a few subjects, which may be due to TC’s strong anti-glycation and glycation reversal effects.
Lastly, TC is allowed for use globally due to its natural status. Taken together, the science developed and the pre-clinical study results observed certainly demonstrate the potential of TC to become a key new topical ingredient for skin care and dermatological products worldwide.
Acknowledgements: The authors wish to thank Sytheon Ltd., for funding this research, and Krys Bojanowski, Ph.D., of Sunny Biodiscovery in Santa Paula, California, for elegantly designing and carrying out the glycation reversal studies reported here.
References
- DV Surya Prakash, SN Sree, S Avanigadda and M Vangalpati, Pharmaceutical review on Terminalia chebula, Intl J Res in Pharma Biomed Sci 3 679–683 (2012)
- R Rathinamoorthy and G Thilagavathi, Terminalia chebula—Review on pharmacological studies, Intl J Pharm Tech Res 6 97–116 (2014)
- W Mullen, B Nemzer, B Ou, J Hunter, N Clifford and E Combet, J Agric and Food Chem 59 3754–3762 (2011)
- U Palanisamy, CH Ming, T Masilamani, T Subramaniam, LL Teng and AK Radhakrishnan, Rind of the rambutan, Nephelium lappaceum, a potential source of natural antioxidants, Food Chem 109 54–63 (2008)
- WL Holtman, JC Vredebbregt-Heistek, NF Schmitt and I Feussner, Lipooxygenase-2 oxygenates storage lipids in embryos of germinating barley, Eur J Biochem 248 452–458 (1997)
- YJ Suzuki, M Tsuchiy and L Packer, Lipoate prevents glucose-induced protein modifications, Free Rad Res Commun 17 211–217 (1992)
- J Dobak, J Grzybowski, FT Liu, B Landon and M Dobke, 1,25-Dihydroxyvitamin D3 increases collagen production in dermal fibroblasts, J Dermatol Sci 8 18–24 (1994)
- H Zhao, A Alexeev, E Chang, G Greenburg and K Bojanowski, Lycium barbarum glycoconjugates: Effect on whole skin and cultured dermal fibroblasts, Phytomedicine 12 132–138 (2005)
- VN Sumantran et al, Hyaluronidase and collagenase inhibitory activities of the herbal formulation Triphala guggulu, J Biosci 32 755-761 (2007)
- JY Lee, JG Oh, JS Kim and KW Lee, Effects of chebulic acid on advanced glycation endproducts-induced collagen cross-linking, Biol Pharm Bull 37 1162 (2014)
- S Pomerantz, Tyrosine hydroxylation catalyzed by mammalian tyrosinase: An improved method of assay, Biochem Biophys Res Commun 16 188-194 (1964)
- K Ohguchi et al, Gnetol as a potent tyrosinase inhibitor from genus Gnetum, Biosci Biotechnol Biochem 67 663-665 (2003)
- GH Naik, KI Priyasarshni, JG Satav, MM Banavalikar, DP Sohoni, and HM Biyani, Phytocehmistry 63 97-104 (2003)
- B Hazra, R Sarkar, S Biswas and N Mandal, Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia bellerica and Emblica officinalis, BMC Complemenatry & Alternative Medicine 10:20 (2010)
- HY Cheng, TC Lin, KH Yu, CM Yang and CC Lin, Antioxidant and free radical-scavenging activities of Terminalia chebula, Biol Pharm Bull 26 1331-1335 (2013)
- GK Glantzounis, EC Tsimoyiannis, AM Kappas and DA Galaris, Uric acid and oxidative stress, Current Pharma Design 11 4145-4151 (2005)
- H Li, A Samouilov, X Liu and JL Zweier, Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation, J Biol Chem 279 16939-16946 (2004)
- DB Reddy, TC Reddy, G Jyotsna, S Sharan, N Priya, V Lakshmipathi and P Reddanna, Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line, J Ethnopharmacol 124 506-512 (2009)
- GJ Fisher, J Varani and JJ Voorhees, Looking older: Fibroblast collapse and therapeutic implications, Arch Dermatol 144 666–672 (2008)
- H Pageon and D Asselineau, An in vitro approach to the chronological aging of skin by glycation of the collagen: The biological effect of glycation on the reconstructed skin model, Ann NY Acad Sci, 1043 529–532 (2005)
- H Pageon, Reaction of glycation and human skin: The effects on the skin and its components, reconstructed skin as a model, Pathol Biol (Paris) 58 226–231 (2010)
- GT Wondra, MJ Robert, MK Jacobson and EL Jacobson, Photosensitized growth inhibition of cultured human skin cells: Mechanism and suppression of oxidative stress from solar irradiation of glycated proteins, J Invest Dermatol 119 489–498 (2002)
- A Neumann, R Schinzel, D Palm, P Riederer and G Münch, High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-kappaB activation and cytokine expression, FEBS Lett 453 283–287 (1999)
- G Dai et al, Chronic ultraviolet irradiation causes loss of hyaluronic acid from mouse dermis because of down regulation of hyaluronic synthase, Am J Pathology 171 1451–1461 (2007)
- KS Girish, K Kemparaju, S Nagaraju and BS Vishwanath, Hyaluronidase inhibitors: A biological and therapeutic perspective, Current Medicinal Chem 16 2261–2288 (2009)
- KS Girish and K Kemparaju, The magic glue hyaluranan and its eraser hyaluronidase: A biological review, Life Sci 80 1921–1943 (2007)
- SJ Kim, SA Sancheti, SS Sancheti, BH Um, SM Yu and SY Seo, Effect of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose on elastase and hyaluronidase activities and its type II collagen expression, Acta Pol Pharm 67 145–150 (2010)
- VN Sumantran et al, Hyaluronidase and collagensase inhibitory activities of the herbal formulation Triphala guggulu, J Biosci 32 755–761 (2007)
- SA Rahbar and JL Figarola, Novel inhibitors of advanced glycation endproducts, Arch Biochem Biophys 419 63–79 (2003)
- JW Hartag et al, Effect of alagebrium, an advanced endproduct breaker, on exercise tolerance and cardiac function in patient with chronic heart failure, Eur J Heart Fail 13 899–908 (2006)
- JY Lee, JG Oh, JS Kim and KW Lee, Effects of chebulic acid on advanced glycation endproducts-induced collagen cross-linking, Biol Pharm Bull 37 1162 (2014)
- GE Costin and VJ Hearing, Human skin pigmentation: Melanocytes modulate skin color in response to stress, FASEB J 21 976–994 (2007)
- P Khazaeli, R Goldoozian and F Sharififar, An evaluation of extracts of five traditional medicinal plants from Iran on the inhibition of mushroom tyrosinase activity and scavenging of free radicals, Int J Cosmet Sci 31 375–381 (2008)
- HA Lee, HJ Cho, KW Lee, SS Park, HC Seo and HJ Suh, Antioxidant activities and melanogesis inhibitory effects of Terminalia chebula in B16/F10 melanoma cells, J Food Sci Nutr 15 213–220 (2010)
- A Manosroi et al, Biological activities of phenolic compounds and triterpenoids from the galls of Terminalia chebula, Chem Biodivers 10(8) 1448–1663 (2013)
- ND Das et al, Terminalia chebula extract acts as a potential NF-kappaB inhibitor in human lymphoblastic T cells, Phytother Res 25(6) 927–994 (2011)
- ND Das et al, Proteomic analysis of Terminali chebula extract-dependent changes in human lymhoblastic T cell protein expression, J Med Food 15 651–657 (2012)
- M Crisan et al, Expression of advanced glycation end-prducts on sun-exposed and non-exposed cutaneous sites the aging process in humans, Plos One 8(10) e75003 (2013)
- P Gkogkolou and M Bohm, Advanced glycation end products, Key players in skin aging? Dermatoendocrinol 4(3) 259–270 (2012)