Click through to the June 2019 digital edition to read the complete article.
Restoring hair's fiber integrity requires understanding the structural and chemical composition of hair, along with the chemistry necessary to counteract the damaging effects of bleaching and color treatment processes. The present article reviews these aspects and proposes a bis-PCA dimethicone solution, which acts via novel bonding mechanisms to impart cumulative strengthening in chemically treated hair.
Structure and Function
Hair’s structure is composed of the cuticle, cortex and medulla.1 The cuticle is the outermost protective layer that provides sensory and shine characteristics. The cuticles overlap in a manner similar to roof shingles, thereby protecting the hair from chemical, physical and environmental treatments. The cortex is the major component of hair and is responsible for its mechanical strength and pigment. The innermost layer is the medulla, which may be present or absent throughout the hair fiber.
The structural integrity of hair is due to its specific chemical composition; primarily keratin proteins, as well as lipids and water. The majority is 65-95% protein, which influences hair textures, e.g., curly, wavy, kinky and straight. These proteins are complex natural compounds that contribute to hair's physicochemical properties.
Human hair keratin is unique due to its relatively higher cysteine residue content, compared with skin keratin, which are 7.6% and 2.9%, respectively.2 Higher levels of cysteine residues in hair leads to a higher amount of inter- and intramolecular disulfide linkages, which translates to a durable structure due to covalent bonds.3
Hair is also comprised of 1-9% lipids, which contributes to enhanced conditioning properties such as flexibility, surface gloss and lubricity of hair.3 Lipids in the internal part of hair provide structural reinforcement and rigidity.
Water is another major component that can comprise up to 32% w/w of hair.4 It supports the formation of a network of hydrogen bonds with proteins, thereby influencing the tensile strength, swelling, flexibility and shape of hair, as well as the formation of salt bridges.
Chemical Processing
Bleaching and dyeing are damaging processes that affect the physical, mechanical and surface properties of hair, leading to dry and straw-like properties that make hair frizzy, difficult to style and easily broken.5 During such chemical treatments, much of the hydrophobic lipid is stripped from the surface of hair, leading to increased tangling due to increased friction and surface roughness. Hair bleaching compositions and methods have been discussed thoroughly in the patent literature.6, 7
Furthermore, hair can become permanently discolored after applying high concentrations of oxidizing agents containing strong alkaline agents. The bleaching process is often highly alkaline (pH 9-13), resulting in a dramatic degradation of the external and internal structural integrity of hair, especially after repeated cycles.
Unlike skin, hair lacks regenerative properties. Therefore, it is important introduce components during bleaching and color treatment processes that preserve and maintain hair’s structural integrity. To this end, a microemulsion based on bis-PCA dimethiconea was designed and tested, as shown here, for its ability to impart the desired effects without affecting the efficacy of bleaching and color systems.
Bis-PCA Solution
The described bis-PCA dimethicone ingredient contains pyrrolidone carboxylic acid (PCA), whose functional group is found naturally in human skin and has been thought to be responsible for ubiquitous moisturization. It is an excellent humectant, displaying moisture-binding capacity that is 1.5× greater than glycerin.8
PCA can form hydrogen bonds with water, which is essential to hair because it maintains hydration and a moisturized, healthy appearance and texture. Aside from hydrogen bonds, however, PCA can reconstruct ionic bonds; in the described microemulsion, the PCA groups at the polymer chain ends are fully ionized at an elevated pH and enhance the affinity of the bis-PCA dimethicone to hair. These ionic bonds can be useful for preventing further hair damage when the PCA dimethicone is coformulated with volatile amines and other solubilizers, favoring the exchange of volatile amine with the amino group in proteins of the hair.
This cumulative strengthening of both hydrogen bonds and ionic bonds becomes crucial to supporting the external and internal structure of keratin protein. When the moisturization and hydrophobicity of hair are in balance, conditioning is improved. Thus, to put the capabilities of the bis-PCA dimethicone ingredient to the test, hair was treated with and without it, subjected to several chemical assaults and its sensory traits were assessed.
Bleach Damage Tests
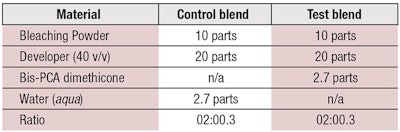
Materials and methods: To test in vitro effects of the ingredient in a bleaching system, virgin black hair tresses were used. These were cut to the same weight and length, then treated with one of two formulas containing bleaching powderb and 40 v/v developer cream (12% hydrogen peroxide). The control formula omitted the microemulsion, whereas the test formula included it at 2.7 parts (see Table 1).
Each formula was brushed onto the virgin tress, wrapped in aluminum foil and allowed to stand at ambient temperature for two hours; tresses were then rinsed with water and blown dry after each application. This bleaching step was repeated for three to four cycles before hair was evaluated.
Note that tresses were not post-treated with conditioners. In addition, the described tests simulated extreme conditions. The goal was to replicate the pre-damaged hair many stylists face when servicing clients, rather than untouched, virgin hair.
Continue reading in the June 2019 digital edition...
References
- Evans, T., and Wickett, R. R. (2012). Practical Modern Hair Science. Allured Business Media, Carol Stream, IL USA.
- Yu, J., Yu, D. W., Checkla, D. M., Freedberg, I. M., and Bertolino, A. P. (1993). Human hair keratins. J Invest Dermatol 101 (1 Suppl) 56S-59S.
- Cruz, C., Costa, C., Gomes, A., Matamá, T., and Cavaco-Paulo, A. (2016). Human hair and the impact of cosmetic procedures: A review on cleansing and shape-modulating cosmetics. Cosmetics 3(3) 26.
- Robbins, C. R. (2012). Chemical composition of different hair types, in Chemical and Physical Behavior of Human Hair. Springer, Berlin, Heidelberg 105-176.
- Gavazzoni Dias, M. F. R. (2015). Hair cosmetics: An overview. Int J Trichology 7(1) 2-15.
- Zeffren, E., and Knohl, R. (1971, Aug. 5). Hair bleaching compositions and process. US Pat 3816614A.
- Edman, W. W., and Sullivan, A. T. (1961, May 31). Hydrogen peroxide hair bleaching composition and method. US Pat 3193464A.
- Romanowski, P. (Accessed 2018, Jun. 13) Humectants—Cosmetic formulating basics. Retrieved from https://chemistscorner.com/humectants-cosmetic-formulating-basics/