Editor's note: In this article, an aqueous, or white propolis, extract was tested in vitro and shown to affect microbiota connected with skin conditions. In vivo trials also demonstrated the ingredient’s safety and efficacy in reducing skin discomfort, dryness and body odor.
Propolis is a resin-like material produced by bees including those of the genus Apis mellifera, also known as the European honeybee. Brown propolis (see Figure 1, below) is perhaps the most well-known, which is transformed by bees from materials collected from poplar and other secondary tree sources such as birch, willow and beech. Composed of resinous and balsamic materials,1 propolis is used to protect, sanitize and insulate the hive; which explains the Greek etymology of pro-polis, meaning "in front of the city.”3, 14
Human health applications: Propolis has been widely embraced by humans to treat bacterial and fungal infections, reduce inflammation and mitigate respiratory conditions.1 It also possesses antioxidant, immunomodulatory, antitumor and anti-ulcer properties. Its topical applications are numerous and include:
- the prevention of photoaging,
- treatment of eczema, psoriasis, fungal infections2-12 and
- wound healing.13
In terms of anti-inflammatory action, studies have shown that propolis enhances innate immunity and modulates inflammatory signaling pathways.13 Also, its antimicrobial mechanism acts by inhibiting the replication process of pathogens and by disrupting their ability to invade host cells. Propolis thereby serves as a barrier against microorganisms and their development.
Active constituents: Propolis is comprised of more than three hundred substances with a predominance of flavonoids; notably galangin, chrysin, tectochrysin, pinocembrin, kaempferol and quercetin. Additionally present are aromatic aldehydes, coumarins, phenolic acids, organic acids and certain trace elements such as aluminum, vanadium, iron, calcium, silicon, manganese, strontium and vitamins B1, B2, B6 and C.
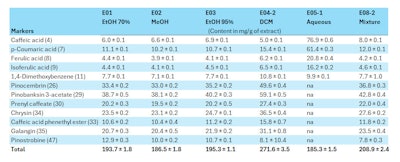
European poplar propolis is particularly rich in flavonoids, phenolic acids and esters (see Table 1, below). To extract them in a form that the human body can absorb, however, requires an effective method to release them from the resin.4, 15-18
Alcoholic vs. aqueous extraction: Indeed, the molecular diversity of a propolis extract can be greatly influenced by the extraction method used, and the presence of certain components in the final preparation depends on their solubility. Hydroalcoholic extracts typically contain the majority of propolis molecules. On the other hand, due to fewer water-soluble components in propolis, non-alcoholic extracts are more difficult to achieve.19
All polyphenolic constituents of propolis have similar therapeutic value. The alcohol in hydroalcoholic extracts can, however, cause formulation issues with other ingredients and potentially irritate skin. Or it may be undesired due to cultural or medical reasons. Aqueous extracts have therefore drawn interest to make propolis-based treatments more accessible, especially for compromised skin.20, 21
As such, an aqueous extraction method based on maceration in spring water was developed that can target the phenolic acid fraction of propolis, yielding an extract rich in caffeic, ferulic and p-coumaric acids.2 These molecules are present in propolis extracts other than European poplar-type propolis, such as Brazilian green propolis,22 and are of great therapeutic interest.
The aim of this study was to evaluate in vitro the potential of the aqueous propolis extract, or white propolisa, to regulate microorganisms associated with skin conditions and other ailments. In addition, assessments were made in vivo to determine the safety and efficacy of dermocosmetic products containing the extract at different concentrations (data proprietary; available upon request).
Microbiota Evolution and Film Formation
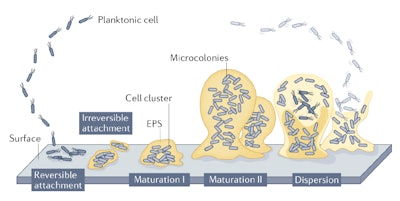
To determine potential interactions between white propolis and microbiota, it is helpful to first understand microbiota behavior in skin. Microorganisms evolve to form biofilms following a five-stage model (see Figure 2, below).26 The planktonic state (stage one – Reversible Attachment) refers to microorganisms suspended in a medium before they adhere to one of two types of surfaces: biotic, referring to living surfaces and tissues including organs, mucous membranes, skin, etc.; or abiotic, including surfaces such as sinks, floors, etc.
After adhesion, they form a biofilm (stage 2 – Irreversible Attachment), i.e., a protective polymeric coating (EPS), to shield them against external threats (such as antibiotics) and promote their proliferation (stage 3 – Maturation I). At the biofilm stage of maturation, attachment becomes irreversible except through mechanical action (e.g., washing with a washcloth and soap, or cleaning surfaces). Maturation continues from cell clusters to microcolonies (step four – Maturation II), following which some disperse (stage five – Dispersion) to detach and form a new biofilm, colonizing the host's surface.27-29
The role of microbiota in the body is essential for many physiological processes, such as protecting the intestinal epithelial barrier, regulating the immunity of the gastrointestinal mucosa, and defending against pathogenic microorganisms.30 On the skin, the complex and constantly evolving microbiota community also contributes to the regulation of skin immunity.
Any changes in this community can compromise the integrity of barrier function and lead to increased inflammatory responses, promoting conditions such as atopic dermatitis, psoriasis, rosacea, seborrheic dermatitis, folliculitis and acne.31 Dysbiosis, which leads to an overgrowth of certain microorganism species at the expense of others, can be caused by external or internal stress, changes in diet or alterations in skin care routines.32
Interest in the skin microbiome has significantly increased in recent years, with research highlighting its crucial role in various processes, such as reducing body odors and protecting against UV radiation. As well, the connection between the gut microbiota, the brain and the skin is increasingly being studied, leading to approaches that combine internal and external care to improve skin health.32-35
Propolis has been shown in various models to significantly affect digestive microorganisms; for example, it has been shown to modulate the flora, improve the mucosal barrier and impart antibacterial activity against pathogens.36-39 It was therefore of interest to study the activity of the white propolis against microbiota associated with skin conditions.
In vitro Materials and Methods
Aqueous propolis extracts/white propolis: Two aqueous extracts of white propolis were prepared by combining raw propolis (various batches from France, Spain, Italy) in water using a reproducible and patented manufacturing process that supports self-preservation.40 The organoleptic and physicochemical quality of the obtained batches was evaluated according to a control plan, and the polyphenol content was measured using the Folin-Ciocalteu method. The average concentration of total polyphenols was 0.18% (g/100 g).
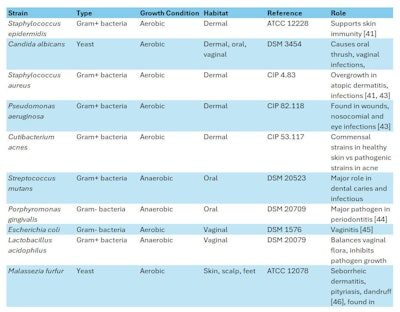
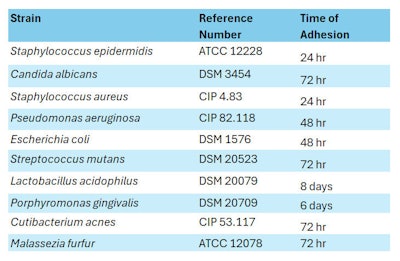
Microbial strains: Ten microbial strains were selected (see Table 2, below) to measure the white propolis extract’s Minimum Inhibitory Concentration (MIC) and to perform Crystal Violet (CV) assays, which relate to the microbes’ planktonic and maturation (biofilm formation) evolutionary phases, respectively. The role of each strain in relation to body site (i.e., dermal, oral, vaginal, scalp, feet) is outlined in Table 2.
MIC measurements: MIC represents the lowest concentration of an active ingredient that completely (100%) inhibits the growth of a microbial strain. It is expressed in μg/mL and determined in a liquid medium (planktonic) by successive dilutions of the tested active compound.
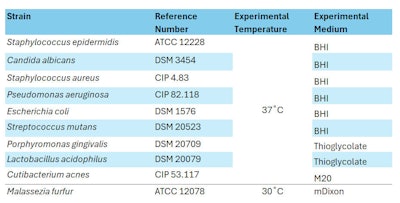
Ten dilutions of white propolis were prepared (2%, 5%, 7.5%, 10%, 15%, 20%, 25%, 30%, 50% and 75% w/v). Each strain was sub-cultured twice from stock solutions to experimental medium agar plates and incubated before the tests in aerobic conditions, except for P. gingivalis, S. mutans and L. acidophilus, which were under anaerobic conditions (see Table 3, below).
One hundred fifty microliters of each product concentration were distributed into three microplates (i.e., three wells per concentration). For microbial inoculation, the initial suspension was prepared in experimental media and the microbial concentration was adjusted at 105 CFU/mL for bacteria and 103 CFU/mL for yeast (Malassezia and Candida) and 50 μL were filled per well.
In parallel, several controls were prepared, including: the strain control (suspension of microorganism + pure water), product control (product + medium) and medium control. The plates were incubated according to the conditions described in Table 3 until a visual growth of the strain was observed. After incubation, the absorbance was measured at 600 nm for each well after shaking the plates.
CV assays: To complement the MIC data and evaluate the ability of the white propolis to inhibit or promote biofilm formation in the tested strains, the total biomass of biofilms was quantified by staining with a dye absorbed by the microorganisms using the Crystal Violet method.
The dilutions of white propolis, incubation concentrations and controls remained unchanged. The same strains were used under identical culture conditions (see Table 3) except for L. acidophilus experimental medium, where thioglycolate was replaced by de Man – Rogosa – Sharpe agar (MRS); and for M. furfur experimental medium, where mDixon was replaced by modified M20.
Again, 150 μL of each product concentration (1.33× concentrate) were filled in the microplates in triplicate. For the L. acidophilus strain, the plates were coated with blood to allow this strain to settle into a biofilm. The plates were incubated under the conditions previously described until reaching a biomass strong enough to be analyzed with CV (see Table 4, below).
After incubation, supernatants were gently removed to eliminate the planktonic cells and reveal the biofilm mass. Biofilms were fixed with ethanol (96%) before dyeing them with Crystal Violet for 30 min. Microwells were then washed with physiological water and acetic acid (33%) was filled in the wells before measuring the absorbance at 630 nm. The higher the optical density (OD630), the higher the amount of biofilm biomass.
White Propolis MIC Results
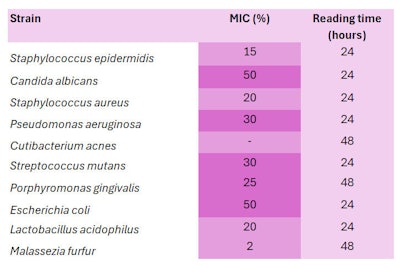
The MICs of the strains tested are listed in Table 5, below. Low MIC values (below 20%) are represented in light purple, while medium MIC values (between 25% and 50%) are represented in dark purple.
Except for C. acnes, an MIC could be determined for all other microorganisms. This indicated the extract, formulated at equal or higher MIC concentrations, had antimicrobial activity on a wide range of bacteria and yeasts.
For example, the white propolis extract inhibited the growth of S. aureus at a low MIC of 20%, and the growth of M. furfur at a very low MIC of 2%. S. aureus overgrowth is implicated in atopic dermatitis and skin infections, while M. furfur plays a role in sebhorreic dermatitis, pityriasis, dandruff and onychomycosis (fungal nail infections).
The white propolis had a more moderate inhibitory activity on E. coli and C. albicans (MIC 50%), which have been implicated respectively in vaginitis, and oral thrush, vaginal infections and onychomycosis. The white propolis extract therefore appeared promising for use as an active ingredient in formulations to regulate microbial imbalances.
White Propolis CV Results
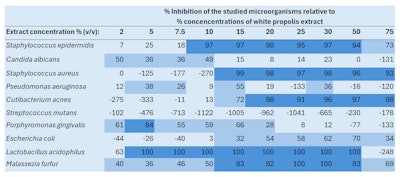
In Table 6, below, CV results are expressed as the percentages at which biofilm formation was inhibited for each of the 10 strains in the presence of white propolis at different concentrations (from 2% to 75%). Results above 80% (dark blue) indicated a total or almost total inhibition of the strains’ biofilm formation. Between 25% and 80% inhibition (light blue), the white propolis partially inhibited biofilm formation. Finally, negative inhibition percentages showed a boost in biofilm formation.
All results were dose-dependent, as the table clearly shows. For example, S. aureus biofilm formation was inhibited by 93-99% at white propolis concentrations of 15% to 75%; at lower white propolis concentrations of 2% to 10%, however, the extract boosted the biofilm formation of S. aureus from 125% to 270%. In the case of M. furfur, all white propolis concentrations (2% to 75%) reduced biofilm formation from 36% to 100%.
MIC, CV Discussion
The results of both analyses showed that where white propolis strongly inhibited planktonic strains (i.e., showed a low MIC), it also strongly inhibited biofilm formation (CV results). This was the case for S. epidermidis, S. aureus, L. acidophilus and M. furfur, suggesting the potential for white propolis to assist in the prevention of acne, atopic or seborrheic dermatitis, dandruff or vaginal microbiota rebalancing, respectively (see Table 2).
Similarly, for strains such as C. albicans, P. aeruginosa, P. gingivalis and E. coli, where white propolis had average MICs (25-50%), a partial inhibition of the biofilm was revealed by the CV analysis. These results suggested that white propolis could potentially help in the prevention of candidiasis infections or periodontitis, aligning with other published studies.49-53
Regarding the results obtained for C. acnes, despite the absence of a measurable MIC, a very good (98%) inhibition of biofilm formation was observed starting with white propolis at 20%. This suggests that, while the extract does not inhibit its proliferation, it can prevent biofilm development. Thus, it may be able to play a role in regulating the proliferation of C. acnes biofilms, helping to rebalance the microbiota of acne-prone skin.
As for the results on S. mutans, despite an MIC of 30%, the CV analysis showed that white propolis impacted biofilm formation, which seemed to slow down and decrease starting at this concentration. The propolis extract therefore showed an ability to regulate the microorganism's capacity to function, even though it permitted an albeit-reduced proliferation. These in vitro studies highlighted the activity of the white propolis extract on strains representative of skin microbiota.
In vivo Effects in Dermocosmetics
The results of the in vitro tests were then compared with in vivo tests (data proprietary; available upon request) evaluating the safety and microbial impact of three dermo-cosmetic products with high concentrations of the white propolis extract (> 99.0%). The products included three sprays: one for atopic skinb, one for sensitive intimate areasc and one for sensitive eyelidsd.
Atopic skin spray: A treatment spray designed for atopic skin to impart soothing effects, relieve sensations of itch and dryness, and control malodor was evaluated for both skin tolerance and deodorant benefits in two tests. The product contained 99.9% propolis extract, with the remainder being water and honey.
The skin tolerance test was carried out under dermatological control in 11 women (mean age ~49 years) with sensitive skin, who applied the test product to the underarm area once daily for 21 days. Results were determined by self evaluations and clinical examinations by dermatologists. The product demonstrated good tolerance, and passed patch testing.
In another 11-volunteer panel (mean age ~41 years), the deodorant efficacy of the spray product was evaluated in a blind sniff test scored by experts. Subjects applied five sprays to a randomly selected armpit; assessments were made 8 hr after application. Deodorant effects were particularly of interest since body odors are often caused by bacteria54-56 and biofilm formation allows them to proliferate and produce even more malodorous molecules.57, 58
The atopic skin spray showed a slight deodorant effect, as measured by clinical scoring, and 82% of volunteers reported a perceived reduction in underarm odor. The observed effects supported the hypothesis that the product may have microbiota-regulating effects (data available upon request).
Intimate spray: A second spray based on the same ingredients as the atopic skin spray (99.9% propolis), designed for sensitive intimate areas to soothe skin, calm irritation and neutralize odor, was evaluated under gynecological control in 22 women (mean age ~45 years). Subjects applied the product to their sensitive intimate areas once daily for 28 days. Results were determined by self evaluations and clinical examinations by a gynecologist to evaluate skin tolerance.
The intimate spray was shown to reduce irritation and discomfort as well as neutralize odor in more than 82% of participants. Furthermore, > 90% also reported less dryness, soothing effects and a reduction in tightness. The product demonstrated good tolerance as well, and passed patch testing (data available upon request).
Eyelid spray: Finally, and eyelid spray designed to reduce puffiness, soothe skin and reduce dryness and flakiness – containing the propolis extract (> 99%), sodium chloride, water propolis wax, pollen and honey – was evaluated under ophthalmologist control in 20 women (mean age ~33 years) having sensitive eye areas.
Subjects applied the product twice daily for 21 days. Results were determined by self evaluation and clinical examination by an ophthalmologist to evaluate skin tolerance. The product was shown to reduce dryness and discomfort in more than 90% of participants (data available upon request). Again, the product also demonstrated good tolerance, and passed patch testing.
Taken together, the results suggested that products containing the white propolis extract could affect skin microbiota in beneficial ways; for example, reducing discomfort and dryness in sensitive skin – which is often linked to the overgrowth of S. aureus.41, 43, 59, 60
Conclusion
This study demonstrated in vitro the effects of an aqueous white propolis extract on skin microbiota proliferation and biofilm formation. In addition, in vivo, the safety, tolerance under medical supervision and perceived effectiveness of products containing high concentrations of the extract were demonstrated (data available upon request), which opens new avenues for additional clinical trials.
Thus, the aqueous propolis could be a promising active for formulations designed to regulate microbial imbalances observed in skin, oral or vaginal flora. Screening a broad spectrum of pathogens showed the most convincing activities were against S. aureus, C. acnes and M. furfur. Thus, by controlling their activities, white propolis could potentially assist in the prevention of acne, dandruff formation or wound healing, respectively.
To enhance our understanding of the action of white propolis aqueous extract on various skin microbiota, the current MIC and CV tests could be supplemented with analyses on microbial strains collected from healthy and unhealthy hosts, or co-culture analyses could be conducted. In vivo double-blind tests on volunteers, with swabbing and microbial gene sequencing analysis, could also be carried out in a future phase.
Gaining a deeper understanding of the described aqueous propolis extract will further efforts to integrate this active ingredient into increasingly effective dermocosmetic treatments, providing efficient solutions for skin issues related to dysbiosis.
Footnotes:
a European poplar propolis, or White Propolis, is made by Ballot-Flurin and is used exclusively in the company’s dermocosmetics. The propolis is produced in compliance with certified organic standards and using Gentle Beekeeping, a method developed by the company to respect bees and other pollinators and to “transcend exploitation.”
b Exyma,
c DermoSpray Intime and
d DermoSpray Eyelids are products of Ballot-Flurin.
References
1. Cordeiro, C.R. (2009). Antibiotic action of propolis on bacteria of the genera Streptococcus, Staphylococcus and Bacillus causing throat infections (in Portuguese). Environmental Management. Available at https://pensaracademico.unifacig.edu.br/index.php/repositoriotcc/article/view/692
2. Boisard, S. (2014, Dec). Chemical characterization and biological valorization of propolis extracts (in French; Caractérisation chimique et valorisation biologique d’extraits de propolis). Doctoral thesis, Université Angers. Available at https://theses.hal.science/tel-01611789v1
3. Ballot-Flurin, C. (2013, April). Apitherapy. Benefits of beehive products (in French; L’Apithérapie. Bienfaits des Produits de la Ruche). Editions Eyrolles. Available at https://www.editions-eyrolles.com/livre/l-apitherapie-bienfaits-des-produits-de-la-ruche
4. Blanc, M. (2010, Nov). Propriétés et usage médical des produits de la ruche. Doctoral thesis, Université de Limoges. Available at https://www.apitherapiefrancophone.com/bibliotheque/2010-proprietes-et-usage-medical-des-produits-de-la-ruche-mickael-blanc-these-en-pharmacie/
5. de Almeida, E.C., and Menezes, H. (2002). Anti-inflammatory activity of propolis extracts, a review. Journal of Venomous Animals and Toxins, 8(2).
6. Bolfa, P., Vidrighinescu, R., … Clichici, S., et al. (2013, Sep). Photoprotective effects of Romanian propolis on skin of mice exposed to 4 UVB irradiation. Food and Chemical Toxicol.
7. Kurek-Górecka, A., Rzepecka-Stojko, A., Górecki, M., Stojko, J., Sosada, M. and Świerczek-Zięba, G. (2014, Dec). Structure and antioxidant activity of polyphenols derived from propolis. Molecules, 19, 78-101.
8. Valero da Silva, M., Gomes de Moura Jr., N., Barretto Motoyama, A. and Moraes Ferreira, V. (2019, Nov). A review of the potential therapeutic and cosmetic use of propolis in topical formulations. Journal of Applied Pharmaceutical Science, 001-011.
9. Bouchelaghem, S. (2021, Dec.). Propolis characterization and antimicrobial activities against Staphylococcus aureus and Candida albicans: A review. Saudi Journal of Biological Sciences, 1936-1946.
10. Pobiega, K., Kras ́niewska, K., Derewiaka, D. and Gniewosz, M. (2019, Sep). Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. Journal of Food Science and Technology.
11. Salatino, A. (2022, Jul). Perspectives for uses of propolis in therapy against infectious diseases. Molecules, 27 4594.
12. Wolska, K., Gorska, A., Antosik, K. and Ługowska, K. (2019, Jul-Aug). Immunomodulatory effects of propolis and its components on basic immune cell functions. Indian Journal of Pharmaceutical Sciences, 575-588.
13. da Rosa, C., Bueno, I.L., Martins Quaresma, A.C. and Barbarini Longato, G. (2022, Sep). Healing potential of propolis in skin wounds evidenced by clinical studies. Pharmaceuticals, 15(9) 1143.
14. Wagh, V.D. (2013, Dec). Propolis: A wonder bees product and its pharmacological potentials. Adv Pharmacol Sci.
15. Chasset, T., Ha ̈be, T.T., Ristivojevic, P. and Morlock, G.E. (2016, Sep). Profiling and classification of French propolis by combined multivariate data analysis of planar chromatograms and scanning direct analysis in real time mass spectra. Journal of Chromatography A, vol. 1465 197-204.
16. Popova, M.P., Bankova, V.S., ... Sabatini, A.-G., et al. (2007, Jun). Chemical characteristics of poplar type propolis of different geographic origin. Apidologie, 38 3 306.
17. de Groot, A.C., Popova, M.P. and Bankova, V.S. (2014, Jun). An update on the constituents of poplar-type propolis. Wapserveen, The Netherlands: AC Degroot Publishing.
18. Huang, S., Zhang, C.-P., Kai Wang, Li, G.Q. and Hu, F.-L. (2014, Nov). Recent Advances in the chemical composition of propolis. Molecules, 19 19610-19632.
19. Bankova, V., Trusheva, B. and Popova, M. (2021, Apr). Propolis extraction methods: A review. Journal of Apicultural Research, 60(5).
20. Kubiliene, L., Laugaliene, V., … Savickas, A., et al. (2015, May). Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complementary and Alternative Medicine, 15 (156).
21. Juodeikaitė, D., Žilius, M. and Briedis, V. (2022, Jul). Preparation of aqueous propolis extracts applying microwave-assisted extraction. Processes, 10(7) 1330.
22. Marcucci, M.C., Ferreres, F., … Antonio, W., et al. (2000). Evaluation of phenolic compounds in Brazilian propolis from different geographic regions. Zeitschrift für Naturforschung C, 55(1-2) 76-81.
23. INRAE. (Accessed 2024, Nov 30). Microbiota, a world of microorganisms (in French). Available at https://tinyurl.com/2wkss5nz
24. Inserm. (2021, Oct 18). Intestinal microbiota (intestinal flora). A serious lead to understanding the origin of many diseases. Available at https://tinyurl.com/2wa3a8zf
25. Cho, I. and Blaser, M.J. (2012, Apr). The human microbiome: At the interface of health and disease. Nature Reviews Genetics, 13 260-270.
26. Sauer, K., Stoodley, P., … Bjarnsholt, T., et al. (2022, Aug). The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nature Reviews Microbiology, 20 608-620.
27. Tremblay, Y.D.N., Hathroubi, S. and Jacques, M. (2024, Apr). Les biofilms bactériens: Leur importance en santé animale et en santé publique. Canadian Journal of Veterinary Research, 78(2) 110-116.
28. Liu, D., Huang, Q., Gu, W. and Zeng, X.-A. (2021, Jan). A review of bacterial biofilm control by physical strategies. Critical Reviews in Food Science and Nutrition, 3453-3470.
29. Alotaibi, G.F. and Bukhari, M.A. (2021, May). Factors influencing bacterial biofilm formation and development. American Journal of Biomedical Science & Research, 12(6).
30. Thursby, E. and Juge, N. (2017, Jun). Introduction to the human gut microbiota. Biochemical Journal, 474(11) 1823-1836.
31. Zhang, X.E., Zheng, P., … Guo, J., et al. (2024, Feb). Microbiome: Role in inflammatory skin diseases. Journal of Inflammation Research, 17 1057-1082.
32. Skowron, K., Bauza-Kaszewska, J., Gospodarek-Komkowska, E., et al. (2021, Mar). Human skin microbiome: Impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms, 9 543.
33. Patra, V., Gallais Sérézal, I. and Wolf, P. (2020, Jun). Potential of skin microbiome, pro and/or pre-biotics to affect local cutaneous responses to UV exposure. Nutrients, 12.
34. Chen, G., Chen, Z.-M., … Chen, J.-G., et al. (2020, Nov). Gut-brain-skin axis in psoriasis: A review. Dermatology Therapy, 11 25-38.
35. Carlomagno, F., Roveda, G., Michelotti, A., Ruggeri, F. and Tursi, F. (2022, May). Anti-skin-aging effect of a treatment with a cosmetic product and a food supplement based on a new hyaluronan: A randomized clinical study in healthy women. Cosmetics, 9(3) 54.
36. Guan, R., Ma, M., Liu, G., Wu, Q., Su, S., Wang, J. and Geng, Y. (2023, Jun). Ethanol extract of propolis regulates type 2 diabetes in mice via metabolism and gut microbiota. Journal of Ethnopharmacology, vol 310.
37. Xue, M., Liu, Y., … Liang, H., et al. (2019, Oct). Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomedicine and Pharmacotherapy, vol. 118.
38. Mohamed Elsherif, H., Orabi, A., Ali, A.S. and Ahmed, S. (2021, Mar). Castor and propolis extracts as antibiotic alternatives to enhance broiler performance, intestinal microbiota and humoral immunity. Advances in Animal and Veterinary Sciences, 9(5) 734-742.
39. Garzarella, E.U., Navajas-Porras, B., … Daglia, M., et al. (2022, Apr). Evaluating the effects of a standardized polyphenol mixture extracted from poplar-type propolis on healthy and diseased human gut microbiota. Biomedicine & Pharmacotherapy, vol. 148.
40. Ballot-Flurin, C. (2018, Jun 6). Propolis processing method. European Pat EP2150127A2. Available at https://patents.google.com/patent/EP2150127A2/fr.
41. Egert, M., Simmering, R. and Riedel, C.U. (2017, Jul). The association of the skin microbiota with health, immunity and disease. Clin Pharmacol Ther. Available at https://pubmed.ncbi.nlm.nih.gov/28380682/.
42. Lopes, J.P. and Lionakis, M.S. (2021, Dec). Pathogenesis and virulence of Candida albicans. Virulence, 13(1) 89-121.
43. Ederveen, T.H.A., Smits, J.P.T., Boekhorst, J., Schalkwijk, J., van denBogaard, E.H. and Zeeuwen, P.L.J.M. (2020, Jul). Skin microbiota in health and disease: From sequencing to biology. The Journal of Dermatology, 47 1110-1118.
44. Sixou, M., Diouf, A. and Alvares, D. (2007). Biofilm buccal et pathologies buccodentaires. Antibiotiques, 9 181-188.
45. Bertuccini, L., Russo, R., Iosi, F. and Superti, F. (2017, Feb). Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. International Journal of Immunopathology and Pharmacology, 30(2) 163-167.
46. Theelen, B., Cafarchia, C., Gaitanis, G., Bassukas, I.D., Boekhout, T. and Dawson, Jr., T.L. (2018, Oct). Malassezia ecology, pathophysiology and treatment. Medical Mycology, 56 S10-S25.
47. Chowdhary, A., Randhawa, H.S., Sharma, S., Brandt, M.E. and Kumar, S. (2005, Feb). Malassezia furfur in a case of onychomycosis: Colonizer or etiologic agent? Medical Mycology, 43 87/90.
48. Andrade Lopes, L.A., Bezerra dos Santos Rodrigues, J., Magnani, M., Leite de Souza, E. and de Siqueira-Júnior, J.P. (2017, Jun). Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microbial Pathogenesis, 107 193-197.
49. Nakao, R., Senpuku, H., Ohnishi, M., Takai, H. and Ogata, Y. (2020, Feb). Effect of topical administration of propolis in chronic periodontitis. Odontology, 108 704-714.
50. López-Valverde, N., Pardal-Peláez, B., ... Manuel Ramírez, J., et al. (2021, Feb). Effectiveness of propolis in the treatment of periodontal disease: Updated systematic review with meta-analysis. Antioxidants, 10(2) 269.
51. Lu, L.-C., Chen, Y.-W. and Chou, C.C. (2005, Jul). Antibacterial activity of propolis against Staphylococcus aureus. International Journal of Food Microbiology, 102(2) 213-220.
52. Przybyłek, I. and Karpiński, T.M. (2019, May). Antibacterial properties of propolis. Molecules, 24 2047.
53. Queiroga, M.C., Laranjo, M., Andrade, N., Marques, M., Rodrigues, A. and Antunes Costa, C.M. (2023, Feb). Antimicrobial, antibiofilm and toxicological assessment of propolis. Antibiotics, 12 347.
54. Wulandari, N.F., Suharna, N., Yulinery, T. and Nurhidayat, N. (2020, Feb). Preliminary study on bacterial diversity causing human foot odor. IOP Conference Series: Earth and Environmental Science, vol. 439.
55. Lam, T.H., Verzotto, D., … Nagarajan, N., et al. (2018, Nov). Understanding the microbial basis of body odor in pre-pubescent children and teenagers. Microbiome, 6(213).
56. Csángó, P.A. (1982, Apr 16-17). First International Conference on Vaginosis: Nonspecific Vaginitis, Kristiansand, Norway. Scandinavian Journal of Infectious Diseases, 14(40) 1-126.
57. Al-Talib, H., Hasan Abdulwahab, M., Murad, K., Amiruddin, N.D. and Ngah Mohamed, N. (2023, Jan). Antimicrobial effects of tetraspanin CD9 peptide against microbiota causing armpit malodor. Antibiotics, 12(2) 271.
58. Edwards-Jones, V.A.L. (2018). Microbiology and malodorous wounds. Wounds, 14(4) 72-75.
59. Cork, M.J. (1996, Jul). The role of Staphylococcus aureus in atopic eczema: Treatment strategies. Journal of the European Academy of Dermatology and Venereology, 7(1) S31-S37.
60. Rippke, F., Schreiner, V., Doering, T. and Maibach, H.I. (2012, Aug). Stratum corneum pH in atopic dermatitis, impact on skin barrier function and colonization with Staphylococcus aureus. American Journal of Clinical Dermatology, 5 217-223.