With the dog days of summer now behind us, we have undoubtedly been reminded countless times of the benefits of wearing sunscreen and the potentially harmful effects of the sun’s UV rays. To this end, it is perhaps surprising that protecting hair against this insult has not become a bigger product proposition.
The sun’s interaction with our skin represents a very serious health risk—and any impact on hair appears trivial in comparison. But nonetheless there appears a well-entrenched general awareness that the sun’s rays also take a toll on the properties of hair.
Here Comes the Sun
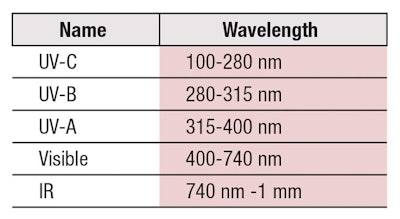
Our sun emits an electromagnetic spectrum of radiation—much of which is harmful to life on our planet. Thankfully, the earth’s magnetic field deflects many of these rays, while others are adsorbed by our atmosphere. Table 1 shows wavelengths of light reaching our atmosphere, although the most harmful—UV-C and some portion of UV-B—radiation is filtered out by the ozone layer.
The energy of these rays is inversely proportional to the wavelength. Much is made of the more energetic nature of UV radiation, but approximately 10 times more visible radiation reaches the earth’s surface. Some sun-related effects are thought attributable to this component.
Any scientific study into the effects of sun on hair necessitates a reproducible means of sun exposure. External weathering is complicated by variation in the sun’s intensity with the seasons—together with other weather-related variables, such as cloud cover, rain, variable humidity, etc. Therefore, attention turns to artificial means of achieving this goal and the availability of accelerated weathering devices.
Accelerated Weathering Chambers
A number of industries require a means of artificial weathering. Commercially-available instrumentation exists but complications arise due to the many variables associated with operation. For example, the use of different lamps—e.g. xenon or fluorescent—represents changing spectral light profiles, which may be further modified by the use of various filters. The intensity, or irradiance, of the light is important, as is exposure time. The relative humidity of the surrounding atmosphere is frequently reported to be a contributor, as is the geometry of the weathering chamber (i.e., the distance between the lamp and the samples).
While a reasonable amount of literature has been generated in this area, there has been no consistency in artificial weathering conditions so it is difficult to compare and contrast historical findings. Descriptions of the weathering process are often scant or, in some instances, completely absent. So while these studies convincingly demonstrate detrimental effects that can be induced by sunlight, a good sense of the magnitude of this damage, in relation to better-understood hair insults (e.g., chemical treatments, heat, etc.), is yet to be determined.
From a Hair's Perspective
Transfer of the sun’s energy to hair necessitates the presence of absorptive species—and it is only the aromatic amino acids that possess electronic transitions equivalent to the wavelength of UV light. The strongest absorber and also the most abundant of these species in hair is tryptophan, and accordingly treatises on the interaction of sunlight and hair generally begin with this molecule (see Figure 1).
More often, discussions on the amino acid composition of hair begin with cystine, which is considerably more abundant and provides extensive contribution to the overall structure. Cystine itself is not especially absorptive to UV light, although many papers document:
- Depletion of this species after weathering1
- An increase in cysteic acid2—the product of cystine oxidation, and/or
- A decrease in mechanical strength of fibers
Figure 2 shows data illustrating diminished tensile strength of hair fibers as a function of accelerated weathering. To explain this occurrence, it is necessary to consider potential pathways for excited tryptophan molecules.
Equation 1, below, shows the absorption of light/energy by a chromophore to induce an energetically excited state. Equation 2, below, shows one potential next step, wherein the excited state simply loses this energy as fluorescence. To this point, it is well-recognized that tryptophan—and hair—does indeed fluoresce after exposure to UV light.3
Equation 3, below, shows a second potential pathway in which the excited chromophore undergoes decomposition. Similarly, reductions in tryptophan levels of hair after UV exposure have been widely reported.3 Tryptophan decomposition can lead to the formation of kynurenines that are believed to be the culprit behind a photo-yellowing process that can be of particular concern for those with grey hair.4
Equation 4, below, shows how the excited species may transfer this energy to another species. Cystine is well-recognized as an effective quencher molecule. Accordingly, Equation 5, below, shows how excited cystine is created, while Equation 6, also below, shows its subsequent breakdown into cysteic acid.
A + hγ → A* Eq. 1 excitation
A* → A + hγ Eq. 2 fluorescence
A* → B + C Eq. 3 decomposition
A* + Q → A + Q* Eq. 4 quenching
Trp* + Cys → Trp + Cys* Eq. 5
Cys* → 2Cys-COOH Eq. 6
The Influence of Melanin
Melanin is nature’s sunscreen; but perhaps surprisingly, it does not have especially strong UV absorptive capability.3 Heavy pigmentation associated with high melanin concentrations provides some attenuation of light penetration into hair fibers; but melanin too is known as a quencher. Therefore, as shown in Equations 7 and 8, below, melanin molecules can acquire this absorbed energy to become excited and subsequently undergo sacrificial decomposition in place of more-important proteins.
Trp* + Melanin → Trp + Melanin* Eq. 7
Melanin* → Decomposition products Eq. 8
Sun lightening of hair is visible evidence for this photo-induced breakdown of melanin. With this said, a convincing number of historical papers have concluded that visible light provides a high contribution to this photo-lightening.5 These publications suggest the UV portion of the spectrum has greater impact on the protein structure, while visible light has a bigger impact on color fading.
Lightly pigmented hair containing low levels of melanin receives little of this protection against the sun’s rays; higher levels of photo damage have been widely reported for these hair types.1
Sun Protection for Hair
The idea of a Hair Protection Factor (HPF)—similar to skin’s SPF—was suggested by Nacht6 in 1990. This idea presents attractive marketing possibilities in an area where quantitative claims are especially popular, yet this proposition is not as straightforward as perhaps presumed.
Several measurement approaches can quantify changing hair properties with sun exposure, which may therefore be used to evaluate product-related benefits in mitigating such processes. However, such techniques relate to specific and often different portions of hair’s complex structure and may therefore yield differing conclusions. For example, Figure 2 showed the decrease in the wet-state tensile strength of the hair as a function of weathering, a property that is widely-attributed to the integrity of crystalline, alpha-helical keratin microfibrils within cortical cells.
Another frequently used measure of hair damage involves quantifying the denaturing temperature of this protein structure by Differential Scanning Calorimetry (DSC)7—an occurrence that is dictated by the state of the surrounding amorphous matrix protein. A simple approach may be to measure photo-lightening of the hair,8 which specifically relates to melanin pigment. Another alternative may be to spectroscopically measure cysteic acid levels in the outer cuticle. Figure 3 shows sample spectra illustrating the presence of a characteristic peak at 1060 cm-1, related to the presence of this species.
Further compounding this issue, the severity of the sun’s effects on hair will be dependent on the level of pigmentation. Differing magnitudes of response to these measures would be expected when weathering dark and lightly colored hair. The exception would be surface measurements of cysteic acid, as the cuticle does not contain melanin and therefore receives no protection.
In short, it is overly simplistic to evaluate the effects of sun on hair, and any potential product or ingredient-related protection, using a single measurement.
Summary
Numerous articles in the scientific literature document photo-induced changes in the properties of hair. Arguably, the most obvious change is photo-lightening, which can be desirable for those seeking natural highlights but a source of considerable frustration for others.
More worrisome is an ability to photo-oxidize hair protein to produce damage symptoms comparable to those seen with chemical oxidation (i.e. bleaching, coloring, perming). However, inconsistency and difficulty in selecting artificial weathering conditions makes it problematic to ascertain the severity of sun-related insults relative to conventional chemical treatments.
Sunscreens are generally chemicals with high UV absorptive capacity that subsequently lessen the exposure of a given substrate to these harmful rays. Data pertaining to the effectiveness of such ingredients on hair is available, but, in part, this efficacy depends on coating thickness—and too much of these oily materials on the hair surface quickly compromises sensorial properties to unacceptable levels. Therefore, care and attention are required when generating and assessing such data to ensure that acceptable application conditions are used.
The idea of sun protection for hair has been around for some time, but has not generated sufficient interest in the end-consumer to have made much of an impact on the hair care category. This is perhaps surprising given the deluge of new information encountered almost daily on the harmful effects of sun on the skin.
I’m old enough to remember how other attributes, such as mildness, anti-aging and heat-protection floundered in a similar vein before ultimately becoming important to the hair care consumer. So, putting on my soothsayer hat, I predict one day in the not-so-distant future, sun protection for hair will indeed become a sought-after consumer proposition.
The good news is, hair care products are exempt from recent sunscreen regulations, as application occurs on a biologically inert material and any treatment relating to this substrate is considered purely cosmetic. Nonetheless, the monograph does recommend any such claims should specifically be noted as applying to hair protection and not leave for any assumption that the product could be used on skin.
Acknowledgements: The data in Figure 3 was generated by Samuel Gourion-Arsiquaud, principal scientist at TRI-Princeton.
References
1. E Hoting, M Zimmermann and S Hilterhaus-Bong, Photochemical alterations in human hair. 1. Artificial irradiation and investigation of hair proteins, J Cosmet Sci 46 85-99 (1995)
2. C Dubief, Experiments with hair photodegradation, Cosm & Toil 107 95-102 (1992)
3. J Jackowicz and RL McMullen, Tryptophan fluorescence in hair–Examination of contributing factors, J Cosmet Sci 62 291-304 (2011)
4. JM Dyer, JE Plowman, GL Krsinin, S Deb-Choudhury, H Koehn, KR Millington and S Clerens, Proteomic evaluation and location of UVB-induced photo-oxidation in wool, Photochem Photobiol 98 118-127 (2010)
5. T Takahashi and K Nakamura, A study of the photolightening mechanism with visible and ultraviolet light, J Cosmet Sci 55 291-305 (2004)
6. S Nacht, Sunscreens and Hair, Cosm & Toil 105 55-60 (1990)
7. FJ Wortmann, C Springob and G Sendelbach, Investigations of cosmetically treated human hair by DSC in water, J Cosmet Sci 53 219-228 (2002)
8. TA Evans, Quantifying hair color fading, Cosm & Toil 130(1) 30-35 (Jan/Feb 2015)