
PEG/PPG dimethicone is a commonly used raw material in the formulation of personal care products. Today’s question specifically considers: How stable is PEG-8 dimethicone in water over a range of pH values?
Here, Tony O'Lenick explains using data provided from a published report by OSi Specialties entitled, "Hydrolytic Stability of Silsoft Dimethicone Copolyols," by Pigeon, et al. According to O'Lenick, "This report, written years ago, provides valuable insights into the stability and formualatability of PEG/PPG dimethicone. I am not aware of any more insightful work on this important topic."
PEG/PPG Dimethicone: Structure and Properties
O'Lenick writes: PEG/PPG dimethicone is a class of amphiphilic silicone polymers that has a water-soluble and a silicone-soluble group present in the same molecule; Figure 1 shows the structure of the PEG-8 dimethicone evaluated in the following discussion.
Figure 1. PEG-8 Dimethicone
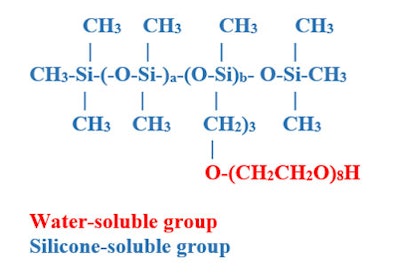 The relative percentage of the weight of the molecule that is water soluble will be a factor in the determination of important properties such as water solubility and surface tension; it will be water insoluble if 20% or less EO w/w; dispersible if 20-50% EO w/w; or water soluble if 50% or more EO w/w.
The relative percentage of the weight of the molecule that is water soluble will be a factor in the determination of important properties such as water solubility and surface tension; it will be water insoluble if 20% or less EO w/w; dispersible if 20-50% EO w/w; or water soluble if 50% or more EO w/w.The identified paper provides significant data related to the effect of molecular weight and pH on the long term solubility in water of the PEG/PPG dimethicone. Specifically, the paper looked at the time it takes to hydrolyze 5% and 20% of the PEG/PPG dimethicone polymer as a function of concentration—1% by weight and 5% by weight. The significant hydrolysis number for the study was the 20% hydrolyzed. The following experimental section details the methodology used for the evaluation.
PEG-8 Stability Experimental*
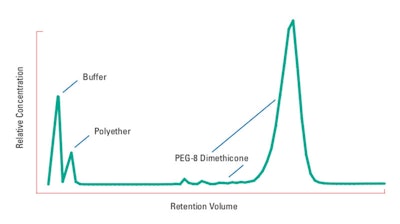
To determine the stability of PEG-8 dimethicone, solutions were prepared by dispersing the copolyols in standard buffer solutions at various pH levels and concentrations. High Performance Liquid Chromatography (HPLC) was used to monitor the stability of the copolyols. Reverse-phase HPLC is an ideal method for separating dimethicone copolyols from the other components in the buffer solutions. A typical HPLC chromatogram of a PEG 8 dimethicone is shown in Figure 2; results of the described evaluation are provided in Table 1 and the data on some properties of the polymer are shown in Table 2 (see Tables below).
Figure 2. Typical reverse-phase HPLC chromatogram of a dimethicone copolyol
 To more conveniently classify the stability of the PEG-8 dimethicone, the percent of unhydrolyzed material was plotted against time. Two points on the curves were used to characterize the stability of the polymer:
To more conveniently classify the stability of the PEG-8 dimethicone, the percent of unhydrolyzed material was plotted against time. Two points on the curves were used to characterize the stability of the polymer:95% unhydrolyzed: The amount of time over which there is no hydrolysis; and
80% unhydrolyzed: The amount of time at which a hydrolysis rate trend has been established.
Methodology: Waters Alliance 2960 HPLC Chromatograph Alltech 500E Evaporative Laser Light Scattering Detector with Low Temperature Adapter (ELSD/LTA); conditions = 35°C 1.95 SLPM N2 carrier column = Phenomenex LUNA C18 end cap, 5 micron, 75 x 4.6 mm; gradient = water/methanol/isopropanol at 1.0 mL/min. Solutions were injected without dilution. Injection volumes ranged from 5 mL for 5.0% solutions to 20 mL for 0.5% solutions.
Table 1. Time to Reach 95% and 80% Unhydrolyzed Material for a PEG-8 Dimethicone* at Differen pH Levels and Concentrations
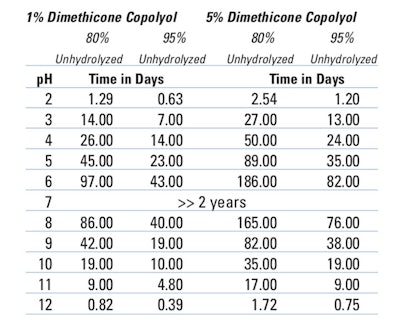 Table 2. PEG-8 Dimethicone Tested in Table 1, Above
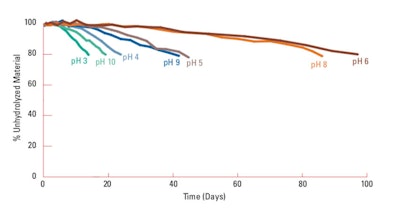
Table 2. PEG-8 Dimethicone Tested in Table 1, Above Figure 3 shows the hydrolysis as a function of pH over time.
Figure 3 shows the hydrolysis as a function of pH over time.Figure 3. Percent unhydrolyzed material vs. time; a PEG-8 dimethicone at various pH levels (at 1.0%)
 Conclusions
ConclusionsThe authors' work shows:
- The functional pH range for PEG-8 dimethicone, to provide stable formulations, is between pH 5.5–8.5.
- At a pH of 5 and 1% active, it took 45 days for 20% of the PEG-8 dimethicone to hydrolyze.
- At a pH of 8 and 1% active, it took 86 days for 20% of the PEG-8 dimethicone to hydrolyze.
- At a pH of 5 and 5% active, it took 89 days for 20% of the PEG-8 dimethicone to hydrolyze.
- And, at a pH of 8 and 5% active, it took 165 days for 20% of the PEG-8 dimethicone to hydrolyze.
Thus, the stability of the PEG-8 dimethicone increases as: 1) the solubility of the PEG-8 dimethicone in water decreases; 2) the molecular weight of the PEG-8 dimethicone increases (> 10,000); and 3) the concentration of the PEG-8 dimethicone increases (from 1% to 5%).
*Adapted from the report by Pigeon, et al.










