Cosmetics, shampoos and most personal care products are typically assessed for ocular irritation throughout the formulation process; however, they are required to be tested before the final product is released in order to provide proper warning labels. Otherwise, the manufacturer must indicate the product has not been tested. In fact, according to the US Food and Drug Administration (FDA), “A cosmetic is considered misbranded if its safety has not been substantiated and it does not bear the following conspicuous statement on the principal display panel (PDP): ‘Warning–The safety of this product has not been determined.’” Starting in 2013, most cosmetics and personal care products sold in Europe also will be required to include safety guidance on their labels.
There are additional compelling reasons to test for ocular irritation. Primarily, products that irritate the eyes will obviously have limited market longevity and put brand reputations at risk. Since consumers can be impacted by even mild ocular irritation, the test used should be highly accurate in its assessment of mild and moderate irritants, as well as severe irritants. Currently it is up to formulators to determine which ocular irritation test is used.
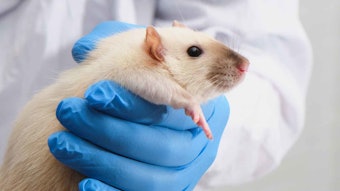
Traditionally, ocular irritation testing has been conducted using rabbits. A test substance is applied to the conjunctival sack of rabbits under restraint for 4 hr, followed by a 14-day test period. After the first hour and on days 1, 2, 3, 4, 7 and 14, the immune response—i.e., redness, swelling and discharge, as well as tissue damage, are evaluated in three areas of the eye: the cornea, iris and conjuntiva. However, the restraint of the animals as well as the 14-day test duration have raised public concern over unnecessary animal suffering.
One purpose of this test is to evaluate any opacity caused by the test substance in the cornea of the eye and to measure it. Any disruption to the pigmented iris surrounding the pupil, which is deeper within the eye than the cornea, is also measured, and the conjunctiva or tissue surrounding the eye is evaluated for redness, chemosis and discharge.3 Different designations are used to classify the various physiological responses—i.e., Draize, European Union (EU), Globally Harmonized System (GHS), US Environmental Protection Agency (EPA), Hazardous Materials Identification System (HMIS), Federal Hazardous Substances Act (FHSA), etc.; using the same animal data but with different weights given to the various responses measured, as well as different overall approaches to statistical analysis.
Ocular irritation is distinguished from ocular corrosion based on reversibility. If, after 14 days, there is still an irritant response, the substance is classified as corrosive, but if the effect has reversed, the substance is classified as an irritant. The irritancy classification systems described above typically include substances that are corrosive within their most severe class—i.e., GHS and EPA “Cat I” or EPA “R41.” Ocular irritation is generally considered a less severe class, i.e., GHS “Cat 2A” and “Cat 2B”; EPA “Cat II” and “Cat III”; and EU “R36.” Nonirritant classes are listed as GHS “NC”, EPA “Cat IV” and EU “NC”.
Ocular Irritation Tests
As previously mentioned, there is broad-based objection and aggressive lobbying against cosmetic and personal care product testing using animals. The “not tested on animals” label appeals to a large segment of consumers. In addition, US regulators discourage animal testing for ocular irritation, and Europe will ban ocular testing for most consumer product labeling. Interestingly, at the same time that animal testing will be banned in 2013, Europe also will mandate safety guidance labeling; the United States is expected to follow suit.
Note: Content preview only; to access the complete article, subscribe to the FREE digital edition or purchase the print edition.








![A 2019 petition to the House of Commons stated, 'We, the undersigned residents of Canada, draw the attention of the House of Commons ... [that] animal testing is unnecessary to prove the safety of cosmetic products.'](https://img.cosmeticsandtoiletries.com/files/base/allured/all/image/2023/01/animal_testing_ban_canada_dreamstime_m_215632720.63d313232306d.png?auto=format%2Ccompress&fit=crop&h=191&q=70&rect=0%2C73%2C1800%2C1013&w=340)
