Editor's note: This two-part article examines the effects of temperature and pH on free-formaldehyde levels (as detected by C-13 NMR) first in water, then in personal care formulations.
Formaldehyde-donor (FD) chemistry represents a class of preservatives commonly used for the control of microbial growth in personal care products, ranging from skin care, moisturizers and cleansers to shampoo and conditioners. Effective over a wide pH range and compatible with most leave-on and rinse-off formulations, FD chemistry has a long history of safe and effective use in personal care and cosmetics products.1 As scrutiny of these and other preservative technologies has increased in recent years, however, additional assurances of the relative safety and benefits of FD technologies are required.
Measuring the amount of free anhydrous formaldehyde (FFD) in equilibrium with bound formaldehyde in a personal care formulation subjected to various pH levels and temperatures generally can determine the stability of an FD preservative technology. A number of analytical methods exist to measure the FFD concentration. These include wet chemical “batch” methods, whereby derivatization agents such as acetylacetone are used to form a colored compound; and chromatographic methods, such as the Engelhardt-Bore method (EB method), which is a post column derivatization method.2
However, almost all of the methods and many of the analytical reagents upset the equilibrium between free and bound formaldehyde, either by releasing or depleting bound or free formaldehyde. These equilibrium upsetting methods may yield results that do not represent the actual levels of FFD in complex personal care formulations.
The present researchers used carbon-13 nuclear magnetic resonance spectroscopy (C-13 NMR) to mirror the FFD levels occurring in stable personal care products. This approach analyzes levels of free formaldehyde and other related compounds, such as methylene glycol and bound formaldehyde, in a manner without upsetting the equilibrium between bound and free formaldehyde. Low-energy excitation with a radio-frequency pulse, followed by detection of the sample response, yields the chemical composition in a quantitative manner.
The large window of the C-13 NMR spectrum—i.e., up to 220 ppm, provides excellent resolution and is selective by detecting carbon atoms from all the other molecules in the sample. This allows analyses of complex mixtures without interference from other components. Moreover, it requires no sample preparation. Accordingly, the sample is unaltered and unlike many other methods, this allows the analysis of the sample under many different conditions, e.g., temperature, pH and concentration, with a sensitivity to 20 ppm and a high spectral resolution.
Previously, a series of experiments was conducted to demonstrate the accuracy of this method in determining the levels of FFD from the amide-based formaldehyde donors imidazolidinyl urea, diazolidinyl urea and DMDM hydantoin.3 Similar studies have been conducted by other groups.4 The present study examines the effects of temperature and pH on FFD levels as detected by C-13 NMR in water and personal care formulations.
FFD in Water at Varying pH and Temperature
From general chemistry principles, it is known that a complex chemical equilibrium is sensitive to pH, temperature and concentration. Several experiments were therefore conducted to determine the FFAs at different pH levels and temperatures while maintaining the formaldehyde-donor preservative concentration at recommended use levels, specifically:
- diazolidinyl urea at 0.3%,
- DMDM hydantoin at 0.4%, and
- imidazolidinyl urea at 0.6%.
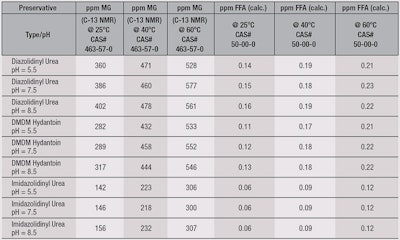
The results are summarized in Table 1.
Overall, these experiments show that increasing the temperature from 25°C to 60°C resulted in increased levels of free methylene glycol and consequently the level of FFD. Similarly, increasing the pH from 5.5 to 8.5 resulted in a small rise in the levels of methylene glycol and FFD. Imidazolidinyl urea, in particular, exhibited roughly half the level of FFD (120 ppb) than the other two preservatives.
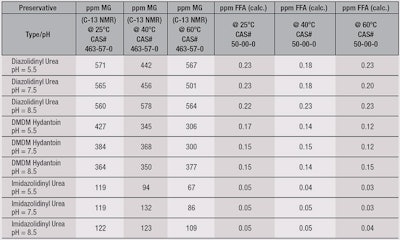
To examine the stability of the formaldehyde donors, the samples were heat-aged from 25°C to 60°C over a one-month period, then analyzed for their FFD distribution at 25°C. The results, summarized in Table 2, indicate all three preservatives were stable across the pH and temperature ranges studied.
Furthermore, imidazolidinyl urea exhibited the lowest level of FFD, present at a level of 50-30 ppb. At this level, methylene glycol approaches that of foodstuffs, such as shellfish (100 ppm).5
(continue to Part II)
References
1. J Krowka, The importance of formaldehyde-donor preservatives in personal care products, Cosm & Toil 128(7) 474 (2013)
2. H Engelhardt and R Klinkner, Determination of free formaldehyde in the presence of donators in cosmetics by HPLC and post-column derivatization, Chromatographia 20(9) 559-565 (1985)
3. M Tallon, JJ Merianos and S Subramanian, Non-destructive method for determining the actual concentration of free formaldehyde in personal care formulations containing formaldehyde-donors, SÖFW 135 22–32 (2009)
4. D Emeis, W Anker and K-P Wittern, Quantitative 13C NMR spectroscopic studies on the equilibrium of formaldehyde with its releasing cosmetic preservatives, Analytical Chemistry 79 2096–2100 (2007)
5. Forma Care (CEFIC) Scientific Fact sheet, Status 1-5 (Oct 21, 2005)







!['[Sunscreen] developers will be able to innovate more efficiently while maintaining high standards of quality and safety for consumers.'](https://img.cosmeticsandtoiletries.com/files/base/allured/all/image/2024/06/woman_outside_using_sunscreen_on_face_ISO_test_standards_AdobeStock_783608310.66678a92029d9.png?auto=format%2Ccompress&fit=crop&h=191&q=70&rect=62%2C0%2C2135%2C1200&w=340)