Editor's note: This two-part article examines the effects of temperature and pH on free-formaldehyde levels (as detected by C-13 NMR) first in water, then in personal care formulations.
FFD at Different Temperatures in Finished Formulations
C-13 NMR analysis was performed as described by Tallon et al.2 on the same preservatives in two sets of personal care formulations: a nonionic moisturizing lotion and gentle facial cleanser.
The preservative level was adjusted in both formulations to:
- diazolidinyl urea at 0.3%,
- DMDM hydantoin at 0.4%, and
- imidazolidinyl urea at 0.6%.
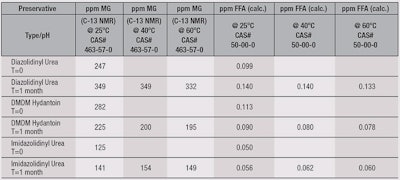
Nonionic moisturizing lotion: Despite the slight increase in the level of MG for imidazolidinyl urea and diazolidinyl urea after one month at 25°C (see Table 3), the temperature stability study revealed a modest decline in FFD for all the formaldehyde donors after aging one month at elevated temperatures, from 40°C to 60°C, in the nonionic moisturizing lotion. Therefore, these preservatives were stable in such a formulation. Additionally, the level of FFD was well below 150 ppb for all the preservatives tested.
Imidazolidinyl urea exhibited the lowest level of FFD among the preservatives, averaging 60 ppb. This is similar to the observation of this preservative in water, which was 50 ppb. More importantly, however, the C-13 NMR analysis demonstrated complex formulations could be examined even in the presence of a number of UV chromophores. This was perhaps the only disadvantage to the UV method used by Walker.6
The overall conclusion of this study demonstrates the true level of anhydrous FFD is well below 150 ppb in this type of formulation, supporting the overall safety of these preservatives in skin care products.
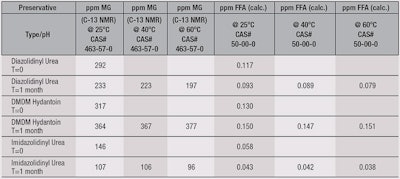
Gentle facial cleanser: In contrast to the nonionic moisturizing formula results, the gentle facial cleanser (see Table 4) exhibited a slight decrease in the level of FFD for imidazolidinyl urea and diazolidinyl urea after one month at 25°C, while DMDM hydantoin increased. Sample aging at elevated temperatures revealed the same trend: FFD levels for DMDM hydantoin rose modestly, from 130 ppb to 150 ppb, while those for imidazolidinyl urea (40 ppb) and diazolidinyl urea (90 ppb) slightly dropped.
Again, these preservatives appear quite stable in this type of formulation. Moreover, imidazolidinyl urea exhibited the lowest FFD levels among the preservatives, averaging 40 ppb, similar to that observed for this preservative in water, which was 50 ppb.
Conclusions
Results from C-13 NMR analysis revealed the actual levels of FFD present from the formaldehyde donors studied in aqueous formulations was three orders of magnitude lower than results from other analytical methods, as shown by Tallon et al.3 These results further corroborate the accuracy and effectiveness of this method for analyzing complex matrices of formaldehyde and formaldehyde adducts.
Here, C-13 NMR spectroscopy determined levels of FFD under different temperatures and pH conditions in water and in complex personal care formulations. An increase in temperature from 25°C to 60°C resulted in an increase level of MG and subsequently FFD in water solutions containing three different formaldehyde donors. Increasing the pH of these water solutions resulted in a small increase in the level of MG and FFD. The level of FFD in the solution containing imidazolidinyl urea was the lowest.
Further, the stability of these formaldehyde donors in two different personal care formulations was demonstrated by heat-aging the products at three different temperatures: 25°C, 40°C and 60°C. In the moisturizing lotion, the levels of FFD slightly declined with heat aging. In a facial cleanser, a slight decline for imidazolidinyl urea and diazolidinyl urea was observed, while DMDM hydantoin showed an increase over time, suggesting the latter was less stable in the facial cleanser.
Due to its non-destructive nature, C-13 NMR spectroscopy is a powerful analytical tool that accurately determines FFD levels in complex matrices. Chemically non-invasive, it is a reliable technique, which could be adopted more widely by the industry.
~ Cosmetics & Toiletries ~
References
2. H Engelhardt and R Klinkner, Determination of free formaldehyde in the presence of donators in cosmetics by HPLC and post-column derivatization, Chromatographia 20(9) 559-565 (1985)
3. M Tallon, JJ Merianos and S Subramanian, Non-destructive method for determining the actual concentration of free formaldehyde in personal care formulations containing formaldehyde-donors, SÖFW 135 22–32 (2009)
6. JF Walker, Formaldehyde 3rd edn, Reinhold Publishing Corp, New York, Table 14, 59-61 (1964)