Several methods and standards have been proposed to determine the protection factor of sun products using indicators such as SPF, UVA protection factor (UVA-PF) and critical wavelength (CW) or broad spectrum efficacy. Although in vivo tests have interesting advantages, in vitro methods are more frequently used for practical, economical and ethical reasons. Recently, several key parameters were identified1–4 for the improvement of in vitro sunscreen testing.
Despite large efforts by the cosmetics industry to determine the photostability of sunscreens, the conditions for UV exposure have not been well-studied. Without strictly controlling the characteristics of instruments, the methods used would hold little value and lack inter-laboratory reproducibility. Unfortunately, even if the curve aspect and level of irradiance is compulsorily checked, external characteristics of irradiation instruments such as the temperature at the substrate surface, beam uniformity and air flow during UV exposure are not yet controlled.
Focusing on these last points, all known parameters were fixed in the study described here, and 22 sunscreens were tested. This work is part of a larger program aimed at reproducibility optimization by identifying, demonstrating and controlling the parameters that influence in vitro UV values on a large selection of products.
Materials and Methods
Solar simulator: Two instruments were used in this study to highlight the importance of controlling external parameters. First, a long-arc xenon solar simulatora (Simulator A) with poor temperature control, poor beam uniformity and uncontrolled air flow was used. Second, a short-arc xenon solar simulatorb (Simulator B) with high temperature control during UV exposure, high beam uniformity and no air flow was used. To comply with the U.S. Food and Drug Administration’s (FDA) final rule for OTC sunscreens,5 a fixed irradiation dose of 800 J/m², an efficient equivalent to 4 MEDs, was used for both instruments.
Sunscreens: Twenty-two products with different properties and cosmetic presentations were selected. Among the samples, five were identified as photo-unstable, eight as thermo-sensitive and nine as sensitive to air flow, as they were presented in liquid form.
Protocol: A strict procedure, described in previous studies, was followed for high reproducibility. First, during the whole process, the temperature at the substrate surface before irradiation was controlled at 25°C using a thermal devicec.1 This appliance also was used with Simulator B for studying the influence of temperature—20–40°C, in 5°C-increments—at the substrate surface during UV exposure. Second, although the FDA’s latest proposal suggests a substrate roughness between 2–7 µm, it has been demonstrated that this large range influences final results.6 Thus, molded polymethylmethacrylate (PMMA) platesd, as recommended in the in vitro UVA-PF method,7 were used for greater reproducibility.
Third, sunscreen was applied at a surface density of 0.75 mg/cm² as nine droplets distributed over the whole surface of the plate with a syringe. Immediately after, the sunscreen was spread in an automated mannere with a pre-saturated finger cot for greater reproducibility, as described in a previous study.3 The sample was allowed to rest for 15 min in the dark at 25°C to ensure self-leveling. Fourth, nine measurements were taken at every 1-nm step from 290–400 nm with a spectrophotometer, which complies with the FDA rule. For each value, two tests were performed for repeatability. Before measurements, the transmittance analyzerf was validated, and the linearity/additivity was calibrated by a reference standardg.
Lastly, with the sunscreen UV transmittance measurement, the in vitro SPF, broad-spectrum efficacy (CW) and photostability percentage (%PS) could be calculated as follows, respectively shown in Equations 1, 2 and 3:
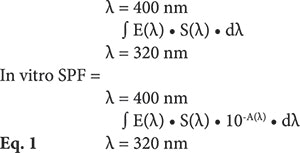
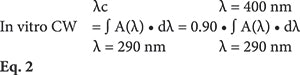

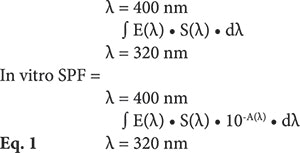
where E(λ) is the erythema action spectrum (CIE-1987); S(λ) is the spectral irradiance, in midday midsummer sunlight; A(λ) is the monochromatic absorbance of sunscreen layer; and d(λ) is the wavelength step, equal to 1 nm in this study.
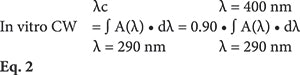
where A(λ) and d(λ) have been previously explained; and λc is the critical wavelength, calculated to comply with Equation 2; and

where SPF has been calculated by Equation 1, before and after UV exposure.
Results: Beam Uniformity
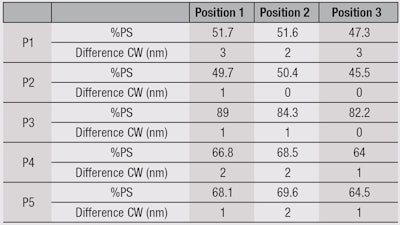
During UV exposure, the temperature with Simulator A at the substrate surface was the same as before UV exposure and as the study conducted without air flow. Results in photostability (%PS) and CW were different before and after UV exposure, as shown in Table 1. To consider variations in beam uniformity, three positions were used in the chamber—two corners, i.e., no. 1 and no. 2; and center, no. 3. These results indicate that changing the positions of the UV irradiation device could lead to different values.
Indeed, position 3, which was in the center of the beam, showed lower photostability percentages, which means the UV dose received in that position by the product was higher than at the two corners. Furthermore, a slight difference between corners was identified, although less than the difference with the center. These interpretations were the same for %PS or CW, indicating that these differences in value could be linked to a lack in beam uniformity.
Results: Temperature at the Substrate
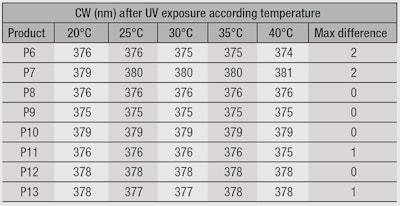
As demonstrated in a previous study,1 controlling the temperature at the substrate’s surface during the whole testing process is necessary to ensure repeatability and reproducibility. Thus, before UV irradiation, the temperature at the substrate surface was maintained at 25°C during the process of application, spreading and drying steps. During the UV exposure temperature evolution from 20°C, 25°C and 30°C, to 35°C and 40°C, the parameters of CW and sun protection, and in turn, %PS, again were studied. Since the photostability percentage depends on the sun protection level, special care was taken to maintain the in vitro SPF very close to that before irradiation for each different temperature during the UV exposure. To focus only on temperature influence, high beam uniformity and no air flow were controlled with Simulator B. The results are expressed by means of CW and %PS, as shown in Table 2 and Figure 1.
The difference in CW, according to the temperature at the substrate surface during UV exposure, was product-dependent. As demonstrated in a previous study,1 for some products, temperature had no influence on CW but in some cases it did—up to 2 nm. Figure 1 clearly shows the influence of temperature at the substrate surface on the %PS, and in vitro SPF values after UV exposition. For several products, a difference of about 5°C led to a difference of 5-10% PS. Furthermore, for some products, this temperature difference could mislead the photostability evaluations. Indeed, for products P7 and P13, the %PS was higher than 100%. This was due to temperature differences and not to UV photostability. Regarding these results, temperature during UV exposure is important. As Simulator B increased the surface temperature by 0.2°C during a 4-MED irradiation, a working range of at least ± 2.0°C during UV exposure should be recommended in the FDA rule and other standards.
Results: Air Flow
Sun protection afforded by sunscreen can be summarized by two points: distribution of intrinsic absorbance due to UV filters, and thickness repartition on the surface of the plate. Through this representation, it seems logical to consider that if, during UV exposure, an external parameter changes the repartition of the product on the whole surface, the value before and after irradiation could also be impacted, leading to a lack of reliability.
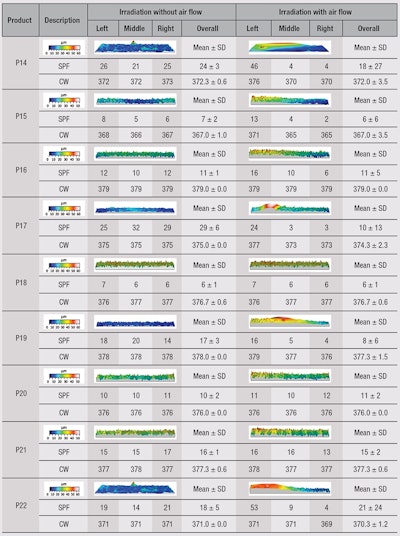
To focus on this parameter, a study was conducted on nine products, with and without airflow, during UV exposure with Simulator A. The temperature was monitored during testing without air flow and adapted during testing with air flow. Also, to understand the influence of airflow on thickness repartition, measurements were taken in three positions on whole substrate surfaces: left, middle and right. Results of UV protection, i.e., in vitro SPF and CW, are shown in Table 3, along with a three-dimensional (3D) overview, as measured by non-contact laser profilometer according to the three different positions and air flow influence.
First, according to the 3D overview, after UV exposure, the samples without air flow showed a consistent film, whereas the samples with air flow presented an irregular film. Second, according to UV measurements in the three different positions, the in vitro SPF and CW were also impacted. Since in vitro SPF is product-dependent, some products measured little in vitro SPF differences with and without air flow, with the exception of standard deviations.
In several cases, however, great differences were observed between the different positions, as was the case for products P14, P17, P19 and P22. Then again, even if CW is based on a balance between UVB and UVA, it can be modified according to measurement area, or at least increase the variability of the mean CW. Based on these results, it is clear that air flow has an impact on in vitro UV protection evaluations due to its modification of thickness repartition.
Indeed, according to the results of the 3D overview and UV measurements, it was observed that the product could be pushed to one side of the plate. This movement leads to greater thickness and UV protection on one side, e.g., the left position, and less thickness and UV protection on other side, i.e., the right position.
Conclusions
In vitro sunscreen tests can reach high levels of reproducibility only when key parameters are strictly controlled, as demonstrated here and in previous studies. Until now, improvements in in vitro methods have not focused enough on controlling external parameters during UV irradiation, which is a compulsory step for UV photostability assessment. This study highlights three parameters as conditions for reliable results: beam uniformity, temperature control at the substrate surface and airflow.
For the first parameter, beam uniformity has been taken into account, but seemingly not enough for a harmonized and reliable method. Indeed, photostability values and in turn, in vitro SPF have shown variability due to poor beam uniformity. Thus, to reduce variability in results, stricter beam uniformity should be required (< 20%), or at least a less total exposure area for acceptance.
The temperature at the substrate surface also should be controlled during the whole process, especially at the step of UV exposure, to improve inter-laboratory tests. Ideally, the same temperature before and after UV exposure is required, as described in recent ISO 24443:2012 standards but clearly, the lower the difference (± 2.0°C), the higher the reliability. Finally, air flow has a great influence on in vitro test values and can lead to misleading information if all phenomena are mixed. Direct air flow on the samples should not be allowed in test methods and standards for reliable results.
References
- S Miksa, D Lutz and C Guy, UV transmission assessment: Influence of temperature on substrate surface, Cosm & Toil 128(7) 484-494 (Jul 2013)
- M Pissavini, S Marguerie, A Dehais, L Ferrero and L Zastrow, Characterizing roughness: A new substrate to measure SPF, Cosm & Toil 124(9) 56-64 (Sep 2009)
- S Miksa, D Lutz and C Guy, In vitro UV testing-robot vs. human spreading for repeatable, reproducible results, Cosm & Toil 128(10) 742-752 (Oct 2013)
- S Miksa, D Lutz and C Guy, Influence of pressure during spreading on UV transmission results, Cosm & Toil 128(9) 822-832 (Nov 2013)
- US FDA, Sunscreen drug products for over-the-counter human use, Final Rule 21 CFR parts 201 and 310, Federal Register 76 (117) (Jun 17, 2011)
- D Lutz, J Ongenaed and C Guy, FDA rule for broad-spectrum labeling: Key substrate findings, Cosm & Toil 126(10) 732-742 (Oct 2011)
- Cosmetics–Sun protection test method–ISO24443:2012 Determination of sunscreen UVA photoprotection in vitro, www.iso.org/iso/catalogue_detail?csnumber=46522 (Accessed Sep 10, 2013)