It is accepted that typical sunscreen application is based on volume, not mass. For example, the Australian Cancer Council recommends that adults apply about a half teaspoon of sunscreen to the face, neck and ears; a teaspoon to each arm and leg; and a teaspoon each to the front and back of the torso.1 However, when the efficacy of sunscreens is evaluated, testing is carried based on a mass, not volumetric, application standard. For example, in vivo SPF and in vitro UVA test specifications require that the sunscreen be applied by mass of the layer per unit area.2–5 Since the attenuation of incident solar radiation through a sunscreen layer at a particular wavelength depends directly on the thickness of the layer (see Beer’s Law), not the mass applied per unit area, this procedure is incorrect and has no scientific basis.
The reason for adopting a testing standard based on mass rather than volume is historical. Until recently, nearly all sunscreens have employed only organic UV absorbers to attenuate UV radiation. Such sunscreens invariably exhibit product densities close to 1 g/cm3. With a density of 1, the application rate of 2 mg/cm2, specified for in vivo SPF testing by the various regulatory bodies,2–4 corresponds to a 2 μL/cm2 volumetric application rate and a uniform 20 micron film thickness. In the past, many studies6–9 as well as the US Food and Drug Administration (FDA)10 have used 2 μL/cm2 and 2 mg/cm2 interchangeably, even referring to thicknesses in units of mg/cm2.
Recently, however, transparent mineral sun care products have increasingly entered the market, which due the high density of their inorganic actives, exhibit densities greater than 1 g/cm3. For example, in Australia there is a rapidly growing market for zinc oxide sunscreens containing 20–25% w/w zinc oxide—and a sunscreen containing 20% zinc oxide has a density approximately 21% greater than a typical organic sunscreen.
The present paper therefore demonstrates that the use of a mass-based application rate for in vivo SPF testing significantly underestimates the in vivo SPF of sunscreens containing high loads of inorganic UV actives, particularly zinc oxide. Further, this supports the notion of a volumetric application rate for in vivo SPF testing, or alternatively, a mass-based application rate adjusted to account for the specific gravity (SG) of the sunscreen.
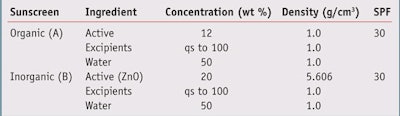
Analysis In the present study, a comparative analysis was conducted of a sunscreen containing organic UV filters and a mineral sunscreen containing the inorganic UV filter zinc oxide. Both sunscreens are assumed to be emulsions containing 50% water and to exhibit an in vivo SPF 30 when measured using the accepted protocol. The values of concentration and density assumed in the analysis are shown in Table 1.
The effect of the UV active concentration on product density is shown in Figure 1. As the concentration of the zinc oxide active increases, the density of the mineral sunscreen increases due to the relatively high density of zinc oxide. In the organic sunscreen, there is no variation in density with concentration since the active and excipient are assumed to have densities equal to 1.
Figure 2 compares the corresponding film thicknesses for the zinc oxide and organic sunscreens for an application rate of 2 mg/cm2. To calculate the film thickness, the application rate (mg/cm2) was divided by the density (g/cm3). Therefore, if the application rate is 2 mg/ cm2 and the density is 1 g/cm3, the film thickness is 0.0002 cm or 20 microns, which is the film thickness for organic sunscreens.
On the other hand, the higher density of the zinc oxide active causes the film thickness to decrease with increasing active concentration. For example, for a concentration of 25% zinc oxide, the film thickness of the zinc oxide sunscreen is only ~80% of that for the organic sunscreen. In other words, the application rate of 2 mg/cm2 divided by 1.26 g/cm3, yielding a film thickness of 0.00016 cm or 16 microns.
For valid SPF testing, it is essential that the applied film thicknesses are the same for all products being tested. The results shown in Figure 2 demonstrate that the standard SPF test protocol of applying the sunscreen at a rate of 2 mg/ cm2 results in the film thickness of zinc oxide sunscreens being significantly reduced, in comparison to sunscreens containing organic actives. And since the SPF of a sunscreen decreases with decreasing film thickness, the measured in vivo SPFs of zinc oxide sunscreens are reduced relative to the values that would be measured at the same film thickness as the organic sunscreen.
Film Thickness and SPF
The dependence of SPF on film thickness is not well-understood. Theoretically, the maximum increase of SPF with increasing film thickness will be given by Beer’s Law only if the extinction coefficient does not vary with wavelength and the absorber concentration and film thickness are spatially uniform— conditions that are never achieved in reality. Both in vivo and in vitro SPF measurements have been conducted on organic sunscreens where the SPF is clearly shown to increase with increasing application rate or film thickness, with both linear and exponential dependencies on film thickness being reported.6, 7, 11, 12 However, no measurements of the effect of film thickness or application rate on in vivo SPF appear to have been reported for inorganic sunscreens.
Herzog has shown13 that the step film model of O’Neil14 provides a basis for calculating realistic values of in vivo SPF from values of molar extinction coefficient, concentration and film thickness. In Figure 3, values of SPF calculated using the O’Neil-Herzog13, 14 model are plotted as a function of applied film thickness for a 20% w/w zinc oxide sunscreen exhibiting an in vivo SPF 30 at an application rate of 2 mg/cm2. Film geometry parameters similar to that used by Herzog13 were employed in the calculation and were assumed to be constant for the range of film thicknesses shown.
As shown in Figure 2, 2 mg/cm2 corresponds to a film thickness of 16.7 μm. To achieve a film thickness of 2 μm, it is necessary to increase the mass loading rate to 2.39 mg/cm2. Using the O’Neil-Herzog step film model, it is predicted that the SPF should increase by 50%, from 30 to 45, when measured in a 20 μm film as compared with using 20 mg/cm2.
Discussion
It is clear from the above example that the current procedure of specifying a mass-based application rate (2 mg/cm2) for in vivo SPF testing is fundamentally incorrect, resulting in erroneous SPF results when applied to sunscreens containing high-density inorganic actives. As a consequence, in vivo SPF values currently reported for inorganic sunscreens may significantly underrate the true level of protection provided by the product when applied to the skin at the same film thickness as an organic sunscreen. In fact, extending the above example to a sunscreen containing 25% w/w zinc oxide would predict an SPF of 50+ if the product were tested at a dosage of 2 μL/cm2.
The incorporation of a volume-based application rate into the relevant test procedures should be straightforward since volumetric pipettes of sufficient accuracy are widely available. Early laboratory measurements often tested sunscreens applied by volume.15–20 Such a procedure may actually improve accuracy as the need to minimize evaporation of the product during application is avoided. Alternatively, a mass-based application rate could be used with the application rate adjusted by the specific gravity (SG) of the sunscreen (application rate = SG x 2 mg/cm2).
This analysis may also be extended to sunscreens containing titanium dioxide as the UV active. Sunscreens containing 8% w/w titanium dioxide with SPFs 30+ are currently available. In comparison to zinc oxide, titanium dioxide has a somewhat lower density (4.23 vs. 5.606 g/cm3). By using the step film model, it is estimated that the SPF 30+ titanium dioxide sunscreen containing 8% w/w titanium dioxide would increase by ~15% if tested at the same film thickness as an organic sunscreen.
Conclusions
The specification of a mass-based product application rate for the in vivo and in vitro testing of sunscreens is fundamentally incorrect and can only be justified if the densities of all sunscreens are the same. With sunscreens containing mineral actives, the density increases with increasing active content, resulting in the average film thickness of the test product to decrease when a constant mass application rate is specified, causing an error in the value of SPF measured. For high mineral loadings, the SPF measured can be significantly less than if it were measured using the same volume application rate as an organic sunscreen.
In conclusion, the product application rate used in current test procedures should be changed from 2 mg/cm2 to 2 μL/cm2 as a matter of high priority to ensure that all sunscreens are tested using the same average film thickness.
Acknowledgements: The author would like to thank Malcolm Nearn, PhD, Innovative Suncare Pty., Ltd.; Takuya Tsuzuki, PhD, Deakin University; and M. Muroi and Jun Chai, Antaria Ltd., for their helpful discussions and comments on this paper.
References
Send e-mail to [email protected].
1. Preventing skin cancer, Cancer Council Australia, www.cancer.org.au/cancersmartlifestyle/ SunSmart/Preventingskincancer.htm (Accessed Jan 14, 2011)
2. Sunscreen products–Evaluation and classification, Australian/New Zealand Standard, AS/ NZS 2604 (1998)
3. Sunscreen drug products for over-the-counter human use; proposed amendments to the Final Monograph, Federal Register 72 165 49070-49122 (Aug 27, 2007)
4. European Commission recommendation of the efficacy of sunscreen products and the claims relating thereto, Official J of the European Community, L265, 7647/EC 39–43 (2006)
5. In vitro method for the determination of the UVA protection factor and critical wavelength values of sunscreen products, Colipa, In vitro Protection Method Task Force Guideline (2009)
6. S Brown and BL Diffey, The effect of applied thickness on sunscreen protection: In vivo and in vitro studies, Photochem and Photobiol 44 509–513 (1986)
7. R Bimczok et al, Influence of applied quantity of sunscreen products on the sun protection factor–A multicenter study organized by the DGK task force sun protection, Skin Pharmacol Physiol 20 57–64 (2007)
8. B Herzog, S Mongiat, K Quass and C Deshayes, Prediction of sun protection factors and UVA parameters of sunscreens by using a calibrated step film model, J Pharm Sciences 93 1780–1795 (2004)
9. B Diffey, Sunscreen isn’t enough, J Photochem and Photobiol B: Biology 64 105–108 (2001)
10. Sunscreen Products for over-the-counter human drugs; Proposed safety, efficacy effective and labeling conditions, Department of Health, Education and Welfare, FDA, USA, Federal Register 43/166 (1978) pp 38206–38269
11. A Faurschou and HC Wulf, The relation between sun protection factor and amount of sunscreen applied in vivo, Photobiol 156 716–719 (2007)
12. L Ferrero, M Pissavini and O Doucet, How a calculated model of sunscreen geometry can explain in vitro and in vivo SPF variation, Photochem and Photobiol Sci 9 540–551 (2010)
13. B Herzog, Prediction of sun protection factors by calculation of transmissions with a calibrated step film model, J Cosmet Sci 53 11–26 (2002)
14. JJ O’Neil, Effect of film irregularities on sunscreen efficacy, J Pharm Sci 73 888–891 (1984)
15. S Thompson, D Jolley and R Marks, Reduction of solar keratoses by regular sunscreen use, N Engl J Med 329 1147–1151 (1993)
16. RM Sayre and PP Agin, Comparison of human sun protection factors to predicted factors using different lamp spectra, J Soc Cosmet Chem 35 439–445 (1984)
17. RM Sayre, PP Agin, GJ LeVee and E Marlowe, A comparison of in vivo and in vitro testing of sunscreening formulas, Photochemistry and Photobiology 29 559–566 (1979)
18. G Groves, PP Agin and RM Sayre, In vitro and in vivo methods to define sunscreen protection, Austr J Derm 20 112 (1979)
19. DF Robertson and GA Groves, Classification of sunscreen preparations, Aust J Derm 18 109 (1977)
20. BL Diffey and J Robson, A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum, J Soc Cosmet Chem 40 127–133 (1989)