Editor's note: Hear Author Commentary on this article from co-author Dr. Fabio Apone in our exclusive podcast.
The human skin, as a physical barrier between the body and outside environment, is subjected to seasonal climate changes that significantly affect its protective functions. The hydrolipidic film that coats the epidermis has key roles in the maintenance of the skin barrier integrity—it lubricates and waterproofs the skin surface, thus preserving an appropriate level of hydration, and protects the inner skin layers from microlesions due to both dehydration and mechanical insults.
In the cold winter season, typical of the Northern Hemisphere, significant decreases occur in all the major lipids of the skin’s hydrolipidic film, including fatty acids, ceramides and cholesterol. These decreases can be observed to the same extent in both the hands and face.1–3 Furthermore, the fatty acid composition and ratio are affected by the cold; e.g., a greater degree of fatty acid unsaturation and decreased levels of palmitic and palmitoleic acids have been associated with an increase of lignoceric acid concentration.4
These alterations have a direct effect on the structure of the stratum corneum, which becomes less compact and loses its mechanical properties. The whole of skin therefore becomes drier and more sensitive, causing discomfort and tightness. Indeed, dermatitis is more prevalent during cold months,5–8 characterized by skin barrier disruption and the release of various pro-inflammatory mediators, generating skin redness due to inflammation and tissue lesions.9–11 At this point, the capacity of skin to repair lesions mainly relies on the activation of coordinated events, including the migration of undamaged cells from wound margins, the synthesis of new extracellular matrix (ECM) components, as well as ECM maturation and remodeling.12
Another relevant consequence of cold temperatures is a decrease in the amount of blood that reaches the skin: at internal body temperatures below 37°C (98°F), skin blood flow is reduced as a physiological response by the body to reduce heat loss and protect against hypothermia.13 Blood delivery through the skin is guaranteed by a complex network of blood vessels in the dermis and the hypodermis, which terminates in thin vascular structures (capillaries). The membranes of the endothelial cells forming these capillaries are responsible for the correct exchange of substances between the skin cells and the blood. During cold exposure, however, the permeability of capillary membranes in the skin is reduced, compromising the supply of nutrients and oxygen to skin cells.14
As such, treatments aimed at the damage in skin produced by cold stress must work on multiple fronts. On one side, to hydrate the skin, making it more resistant and responsive to mechanical injuries; on the other side, to increase the natural supply of nutrients to the skin, to prevent any slowing of cell metabolic activity and cell suffering. These attributes for skin treatment can take inspiration from sessile plants living in cold environments, which have evolved by producing defensive secondary metabolites to survive.
Daphne odora, belonging to the family of Thymelaeaceae, is of particular interest because it well-tolerates low temperatures, even producing heavily perfumed blossoms during the winter season when most of the plants are quiescent. This is possible due to the presence of specific secondary metabolites, which defend the cells against cold stress, prevent tissue damage and actively stimulate cell metabolism.15, 16 Phytochemical studies have shown Daphne species contain a wide range of compounds, including flavonoids, lignans and terpenoids,17 which have various biological activities including anti-inflammatory, antioxidant, analgesic, anticancerogenic and antimicrobial.18–23 Recent studies also demonstrate the wound-healing activity associated with certain Daphne extracts, mostly attributed to the presence of the active compound luteolin-7-O-glucoside.24
On the basis of these findings, cell suspension cultures from Daphne odora plants were developed, from which a hydro-soluble extract, containing lignans and flavonoids—particularly flavons and flavans—was prepared. This extract was tested in vitro on skin cell cultures to determine potential toxicity and effects on sebum production, skin restructuring, wound healing, inflammation and blood flow; and in vivo on human skin to assess its ability to counteract the negative effects of cold stress.
Daphne odora cell culture extract stimulated the production of hydrolipidic film components by skin cells, reducing micro-lesions and inhibiting inflammation.
Test Materials
Callus production, cell cultivation and extract preparation: Leaves of Daphne odora, var. aureomarginata, were surface-sterilized in 70% EtOH for 15 min and 1% bleach for an additional 15 min, then washed three times with sterile water. Each leaf explant was cut into 0.5 cm pieces, wounded with a scalpel blade and placed on Gamborg B5 mediuma, supplemented with 500 mg/L myo-inositol, 30g/L sucrose, 1 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), 0.1 mg/L kinetin, 1 mg/L adenine and 7.5 mg/L plant agar for callus induction. White and friable callus was obtained after five weeks of cultivation in the dark. The callus cultures were maintained by transferring the tissues onto fresh medium every four weeks.
To initiate stem cell liquid culture, 50 mg of 40/45-day-old callus was suspended in 25–30 mL of liquid medium. The culture was incubated at 27°C in the dark under constant orbital stirring (110 rpm). The callus tissue gradually disintegrated and formed single cells or small cell aggregates after 10 days. The suspensions were then transferred into larger volumes, where 50 mL of a dense culture was used as the starting material to inoculate 1 L of culture medium.
After seven days, the cells were collected, filtered and homogenized in PBS buffer (136 mM NaCl, 2.7 mM KCl, 12 mM NaH2PO4 and 1.76 mM KH2PO4; pH 7.4) at a 1:1 ratio (w/v). The resulting lysate was centrifuged at 10,000 rpm to precipitate the particulate fraction and isolate the soluble components. The supernatant was then collected and lyophilized in order to obtain a stable powder (DoHE) that was easy to dissolve in water or cell culture media for testing.
LC-ESIMS/MS analyses: To characterize the resulting extract, mass spectrometry analyses of DoHE were carried out on a hybrid quadrupole-time of flight (q-tof) premier instrumentb equipped with an electrospray ion source—i.e., liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-SEI MS). Liquid chromatography separation was achieved on a Luna C18 columnc and using 0.1% formic acid in water (A) and acetonitrile (B) as eluents. Compounds elution was achieved using a linear gradient from 1% to 50% of B over 45 min.
Mass spectra were acquired both in positive and in negative ion mode over the 200–800 m/z range. The source temperature was set at 120°C, with a capillary voltage of 3300 eV and cone voltage of 50 eV. MS/MS spectra were acquired for the four most abundant ions observed in each MS spectrum (independent scan mode). Compounds identification was achieved on the basis of the MS and MS/MS data, and by taking into account the available chemical information.
Skin cell cultures: For in vitro tests, immortal human keratinocytesd and human dermal fibroblastse were grown in Dulbecco's Modified Eagle Medium (DMEM), containing 4 mM L-glutamine, 4.5 g/L glucose, 1 mM sodium pyruvate and 1.5 g/L sodium bicarbonate, supplemented with 10% FBS, under 5% CO2 humidified atmosphere at 37°C. Human umbilical vein endothelial cellsf were grown in the endothelial growth medium and supplemented with the reagents provided by the MV2 Kitg.
Toxicity Assay via Cell Vitality
To test for the potential toxicity of DoHE, the cell vitality (or MTT) assay was used, wherein 1 x 104 cells (HaCaT or HDF) were seeded in 96-well plates, grown for 16 hr and treated for 48 hr with different concentrations of DoHE. After treatments, cells were washed with PBS and incubated with 100 µL/well of “reaction buffer” containing: 10 mM Hepes, 1.3 mM CaCl2, 1 mM MgSO4, 5 mM glucose and 0.5 mg/mL of colorimetric substrate MTT [3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide] in PBS buffer at pH 7.4. After 3 hr at 37°C and 5% CO2, cells were solubilized by the addition of a 100 µL of lysis solutionh and the plate was incubated for 4 hr at room temperature. The number of healthy cells was directly proportional to the level of the formazan product created. The developed color was then quantified at 595 nm by spectrophotometerj.
Gene Expression Studies for Skin Benefits
To determine the effects of the test material for potential skin benefits such as sebum production, skin restructuring and wound healing, inflammation and blood flow, various assessments were made in three cell types. 5α-Reductase type 1 (5αR1; for sebum production) and secreted protein acidic and cysteine rich (SPARC) expression (for skin maturation and restructuring in wound healing) were analyzed in HDF; cytokine gene expression was tested in HaCaT (for inflammation); and vascular endothelial growth factor (VEGF) gene expression was assessed in HUVEC (for blood flow).
For all three assessments, the respective cells, i.e., HDF, HaCaT and VEGF, were incubated with DoHE for 6 hr, then processed for RNA extraction using an RNA purification kit according to the manufacturer’s instructionsk. For cytokine expression, the cells were incubated with DoHE for 16 hr then infected with bacteria (S. epidermidis) for 3 hr to induce inflammation before being collected for total RNA extraction.
Reverse transcription polymerase chain reaction (RT-PCR) was performed using gene-specific primers and an oligo-nucleotide internal standard kitm. The PCR products obtained were visualized and quantified with an imaging systemn. The amplification band corresponding to the analyzed gene was normalized to the amplification band corresponding to 18S. The values obtained were finally converted into percentage values by considering 100% as the measure for untreated cell samples.
The gene-specific primers used were:
5α-Red1-Fw: 5'ATGTTCCTCGTCCACTACGG3'
5α-Red1-Rv: 5'GGAGGTACCACTCATGATGC3'
SpO-Fw: 5'ATGAGGGCCTGGATCTTCTTTC3'
SpO-Rv: 5'CGCAGCTTCTGCTTCTCAGT3'
hVEGF fw: 5'TGCATTGGAGCCTTGCCTTG 3'
hVEGF rv: 5'GAAGATGTCCACCAGGGTCT 3'
IL-1β Fw: 5'ATGGCAGAAGTACCTGAGCT3'
IL-1β Rv: 5'AAGGACATGGAGAACACCAC3'
IL-8 Fw: 5'GCCACCGGAGCACTCCATAA3'
IL-8 Rv: 5'CTCTTCAAAAACTTCTCCACAACC3'
TNFα Fw: 5'ATGAGCACTGAAAGCATGATCC3'
TNFα Rv: 5'TCATACCAGGGCTTGGCCTCA3'
Wound-healing Effects
Scratch assay: HDF were seeded in 6-well plates; 16 hr later, a line of cells across the middle of the wells was traced by scratching the bottom using a pipette tip. Floating cells were removed by PBS washing. Culture medium containing 0.5% FBS with DoHE or 2.5 ng/mL of TGFβ1 (as a positive control) was added, and the cells were incubated for 7 hr. Mytomycin C (10 μg/mL) was always included in the medium to prevent cell proliferation. To estimate the relative migration of the cells, for each condition, the unclosed cell-free areas were compared using software at time 0 and 7 hr after treatment.
Actin polymerization: The migration of the cells is mainly mediated by actin: By forming polymers starting from the monomeric form, actin drives the formation of cell membrane projections that propel the cell forward. To measure actin polymerization, HDF were seeded in 24 well-plates at a density of 2x105; 16 hr later, the cells were treated for 15–60 min with the extract or 2 μg/mL of phosphatidic acid (PA; positive control). After treatments, the medium was removed and cells were washed with cold PBS 1X, fixed in formaldehyde 4% for 10 min at 4°C and permeabilized with 0.2% surfactant solutionh in PBS for 30 min. The cells were then incubated for 1 hr in the dark with 0.4 mM Rhodamin phalloidin in PBS 1X, washed twice in PBS 1X and solubilized with 300 μL of methanol. Fluorescence at 540 nm was measured by a multi-well plate readerp.
Fibronectin ELISA: Besides actin, a crucial role in the wound healing process is performed by fibronectin, which coordinates the deposition of new extracellular matrix proteins and promotes cellular adhesion and communication. To measure fibronectin activity, HDF were seeded in 96-well plates at density of 9 x 103 per well and treated with extract or 5 ng/mL of TGFβ1 (positive control) for 72 hr without changing the medium. Each condition was repeated in triplicate. After treatments, the medium was removed and the cells were washed with PBS 1X, fixed in para-formaldehyde 4% for 10 min, washed three times with PBS, and permeabilized with 1% surfactant solutionh for 30 min.
After permeabilization, the cells were treated for 30 min with 0.5% polysorbate-20, 5% bovine serum albumin (BSA) in PBS, and incubated a 4°C with primary goat polyclonal antibody, raised against human fibronectinq diluted 1:500 in PBS 1X, containing 0.5% polysorbate-20 and 1% BSA. Samples were washed three times 16 hr later with 0.5% polysorbate-20 in PBS, and incubated with horseradish peroxidase (HRP) labeled anti-goat secondary antibody, diluted 1:1000 in PBS 1X, containing 0.5% polysorbate-20 and 1% BSA.
One hour later, plates were washed three times with PBS 1X and the amount of fibronectin produced by the cells was measured by a colorimetric reaction, using a 0.5 mg/mL solution of o-phenylenediamine (OPD) and 0.012% H2O2 in citrate buffer 50 mM. The plate was incubated at room temperature for 60 min until a yellow color developed. The absorbance of each sample was measured at 490 nm by a multi-well plate readerp.
Daphne extract was effective in improving skin hydration and reducing water loss.
Clinical Tests
Clinical tests were performed in 20 volunteers, ages 18–65, selected for their particularly dry and cracked facial skin due to cold weather and not suffering from any facial skin diseases. The studies were conducted in Montréalr during a time of year averaging ambient temperatures from -11°C (12.2°F) to -7°C (19.4°F). Twice daily (morning and evening), for 28 days, each volunteer applied a cream containing 0.002% of DoHE on one side of the face, massaging until absorbed; the other side was treated with the placebo. This study was carried out in compliance with the most recent recommendations of the World Medical Association Declaration of Helsinki of ethical principles for medical research involving human subjects, and according to the Colipa Guidelines for the evaluation of the efficacy of cosmetic products.
To evaluate the efficacy of the extract, corneometry was useds and transepidermal water loss (TEWL) was investigatedt at T0—i.e., study entry, immediately before analysis began; and at T1, T14 and T28—respectively 1 day, 14 days and 28 days after application. Corneometry is a scientifically recognized method for the measurement of skin hydration. It is based on the determination of the capacitance of the superficial stratum corneum, which is proportional to its aqueous content.25–28
Both types of measurements were carried out in duplicate on the treated areas for each subject and at each experimental time. Results were expressed as mean percentage differences at T1, T14 and T28, compared with T0. Statistical analyses (t-tests) were performed to confirm the significance of the observed differences.
Results: Toxicity and Cell Culture Characterization
Toxicity: As noted, to evaluate the potential toxicity of DoHE and determine the doses to use in cellular assays, a cytotoxicity test (MTT assay) was performed using scalar concentrations of the extract, ranging from 0.05% to 0.0004% w/v, on HaCaT keratinocytes and HDF. After 48 hr, the cell vitality rates were determined. All tested doses resulted in negative cytotoxicity for both cell lines (data not shown). Therefore, for convenience, the concentrations of DoHE chosen for studies on skin cells were 0.002% and 0.01%.
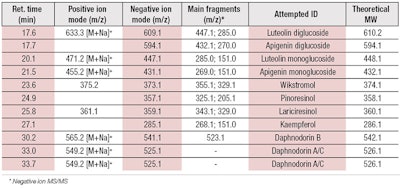
Characterization: DoHE was analyzed for the presence of flavonoids and lignans, which were previously described for Daphne odora and other Daphne species.22, 29 In the present study, chemical analysis revealed the presence of the lignans wikstromol, pinoresinol and lariciresinol, along with three main types of flavonoid compounds: flavonols, such as kaempferol and glucosidic derivatives; flavon glucosides, including luteolin mono and di-glucosides; and flavans, in the form of daphnodorin A, B and C (see Table 1).
Lignans isolated from similar Daphne species have been characterized for their anti-inflammatory activity.23 Also, flavonoids have long been for known for their multiple roles as therapeutic and antioxidant agents;20 thus, they have been used in different types of health care products.30, 31
The results of this chemical characterization led to the exploration for the potential role of DoHE as a soothing and anti-inflammatory ingredient for skin, particularly to alleviate cold stress-related discomfort.
Results: Sebum Production
Sebum comprises a complex mixture of lipids including triglycerides and their derivatives, such as wax esters, squalene, free fatty acid and cholesterol.32 The production of sebum, which is inhibited by cold stress, is mainly regulated by the enzyme 5αR1. This enzyme is produced both by fibroblasts and sebaceous gland cells, and catalyzes the conversion of testosterone to dihydro-testosterone (DHT)—the most active androgen that stimulates sebum biosynthesis.33
To investigate the capacity of DoHE to stimulate sebum production, fibroblasts were treated for 6 hr with the extract and analyzed for the expression of the 5αR1 gene by RT-PCR. The results, shown in Figure 1, indicate treatments with DoHE increased the expression of 5aR1 gene by approx. 50% and 48%, at concentrations of 0.01% and 0.002%, respectively. This suggested a potential role in inducing sebum production via the stimulation of 5αR1 expression.
Results: Wound Healing
Scratch assay: As stated, to test the ability of DoHE to promote the wound healing process, a scratch assay was performed on fibroblasts treated with the extract. In the scratch assay, a “wound gap” in a cell monolayer was created by the scratching and “healing” of this gap by cell migration toward the center of the gap was monitored and quantitated. As shown in Figure 2, the extract increased the capacity of the cells to fill in the wound by accelerating cell migration by approx. 27%, compared with the untreated control. Notably, this effect was even higher than that produced by TGFβ, the positive control.34
Actin polymerization: As described, actin mediates the migration of cells for healing, thus newly polymerized actin was measured in fibroblasts treated with DoHE by using a fluorescent peptide, Rhodamine-phalloidin, which binds specifically to F-actin. As shown in Figure 3a, DoHE treatment increased the production of new polymerized actin by 18% and 8%, at respective concentrations of 0.01% and 0.002%. This suggests the capacity of the extract to stimulate cell migration; PA, as the positive control, confirmed the reliability of these results.35
Fibronectin ELISA: Since fibronectin also plays a role in wound healing, after treating the fibroblasts with DoHE, the amount of fibronectin produced was measured using a goat antibody that acts against it. Treatments with DoHE increased fibronectin production by 15% at both test concentrations (see Figure 3b). The increase was comparable to that produced by the TGFβ, used as the positive control.36
Extracellular matrix (ECM) remodeling: The last key event in the wound healing process is the maturation and remodeling of the ECM. After being secreted by cells, collagen fibers must be rearranged, cross-linked and correctly aligned. To measure this, secreted protein acidic and cysteine rich (SPARC) protein—a collagen-binding protein produced by fibroblasts—can be assessed. This entity plays an essential role in modulating collagen processing and assembly.37, 38 As shown in Figure 4, both test concentrations of DoHE increased SPARC expression levels by 25%, confirming the extract positively regulated ECM protein assembly.
Taken together, these sebum-regulating and wound-healing results indicated the Daphne cell culture extract could potentially improve skin integrity, both by reinforcing the natural hydrolipidic barrier and by accelerating the repair of skin micro-lesions; even acting at different stages of the wound healing process.
Results: Inflammation
Since damage to the skin barrier due to cold stress is often associated with an inflammatory response, the potential of DoHE to inhibit the production of pro-inflammatory cytokines was tested. This was carried out by treating keratinocytes with the extract, and measuring the expression level of interleukins IL-1β, IL-8 and the tumor necrosis factor alpha (TNF-α).39 Parallelly, in the same experiments, the synthetic drug T0901317 was used as positive control.40
As shown in Figure 5, at both concentrations, DoHE significantly inhibited the expression of all the cytokines induced by bacteria infection in the keratinocytes. At the higher concentration of 0.01%, the expression of IL-1β, IL-8 and TNF-α was reduced by approx. 73%, 22% and 43%, respectively. This suggested DoHE had good potential anti-inflammatory activity for the skin.
During cold stress, blood flow and the supply of nutrients to skin cells are reduced.
Results: Blood Flow
As explained above, during cold stress, blood flow and consequently the supply of nutrients and oxygen to skin cells is compromised due to the reduction of endothelial cell membrane permeability. To test the effects of DoHE on membrane permeability in endothelial cells, the gene expression of VEGF was measured.41, 42 As shown in Figure 6, the extract increased VEGF expression in human primary endothelial cells. The highest 0.01% dose of DoHE up-regulated VEGF expression by 66%, suggesting a potential increase in membrane permeability and therefore, possible increase in nutrient delivery to skin cells.
Results: Clinical Tests
To confirm the data obtained by in vitro tests, the activities of DoHE were clinically evaluated in 20 healthy volunteers as described above. For 28 consecutive days, the volunteers applied a test cream containing 0.002% of the extract to the left side of the face, and a placebo emulsion to the right side of the face (see Formula 1). To measure moisturizing efficacy and skin barrier enhancement, hydration levels and TEWL were measured before the product application (T0), and at T1, T14 and T28.
As shown in Figure 7a, hydration increased on average by 21.45% after one day; 34.71% after 14 days; and 62.17% after 28 days with respect to T0. These increases were all statistically significant, as compared with the placebo (p < 0.05). An increase in hydration levels also was observed after the placebo treatment, mainly due to the moisturizing activity of the cream components themselves, but it was much lower than that produced by the DoHE cream.
In a parallel set of tests, TEWL was measured, which is directly related to skin integrity and its capacity to retain water. As shown in Figure 7b, the TEWL decreased by an average of 2.52% after one day; 3.61% after 14 days; and 8.04% (p < 0.05) after 28 days of product application, with respect to T0.
A slight reduction in TEWL also was observed after the placebo application, but this variation was not statistically significant (p > 0.05). These results supported the in vitro data and further suggested that DoHE was effective in improving skin hydration and reducing water loss, particularly in skin exposed to cold conditions.
Results indicate the extract could potentially increase membrane permeability, in turn increasing nutrient delivery to skin cells.
Discussion and Conclusions
The present study identified the primary active constituents in a hydrosoluble extract obtained from Daphne odora cell cultures. Then it tested their capacity to counteract negative effects in skin caused by cold stress, in particular those related to sebum production, inflammatory response, healing process and microcirculation. Indeed, species of the genus Daphne have been used for ages in traditional medicine in China, and in tropical parts of Africa for the treatment of various skin conditions including bruises, insect and snake bites, wounds, ulcers and infections.43
In this study, the Daphne odora cell culture extract (DoHE) was characterized and shown to contain lignans and flavonoids, as well as their glucosidic derivatives, that have several beneficial properties for skin cells—especially in relation to counteracting the negative effects of cold conditions on skin. As demonstrated by clinical tests, the extract produced a faster hydration rate and higher protection against moisture loss, compared with skin treated by a placebo cream only. The cellular assays indicated this action was mostly mediated by: the induction of the sebum regulator 5αR1 in fibroblasts; an inhibition of inflammatory cytokines; an increase in the healing rate of epidermis cells; and stimulation of VEGF expression in endothelial cells.
While activity on 5αR1 by any of the components of DoHE has not specifically been measured, the observed anti-inflammatory properties can be associated with lignans and daphnodorin bioflavonoids. Indeed, both lignans and daphnodorins, isolated from different Daphne species, have been studied for their capacity to inhibit nitric oxide production in macrophages.23, 44, 45 Besides their anti-proliferative activity on different cell tumor lines,22 daphnodorins were previously tested for inhibiting chymase-dependent angiotensin II formation,46 being that the isoforms A and B are the most active. Interestingly, this specific activity can be linked to the observed effect of DoHE to increase endothelial cell permeability through VEGF activation, which antagonizes the angiotensin effect of inducing skin capillary vasoconstriction and reducing blood flow.47 Additionally, the wound healing capacity observed in DoHE-stimulated fibroblasts can be associated with the presence of the luteolin-7-O-glucoside, as found by other authors.24
Finally, particular attention is also deserved by the glycoside derivatives of the flavonoids, which have been studied as potential anti-inflammatory agents48 thanks to their higher skin permeation capacity, compared with the aglycated forms49 and ability to inhibit the NF-kB pathway in endothelial cells.50 In agreement with these results, hesperetin-O-glucuronides, flavonoid glycosides structurally related to both luteolin and apigenin glycosides, have been characterized for their ability to lower blood pressure and enhance endothelium-dependent vasodilation by acting through NO-mediated mechanisms and inhibiting NADPH oxidase activity in vascular endothelial cells.51
Plant cell cultures represent a valuable biotechnological tool to produce active compounds for cosmetic application,52 thanks to their versatility and capacity to adapt metabolic activities to different growing conditions. Several examples of the dermatological activities of plant cell culture-derived compounds have been published, each one showing different effects on skin cells that depend mostly on the chemical prevalence of determinate active molecules exerting specific biological effect on gene expression and functions.53–55 Moreover, the chance to modulate the chemical composition of the plant cell culture extracts by growing the cells in the presence of elicitors makes plant tissue cultures unique in terms of flexibility and applicability.56
Acknowledgements: The research was supported by the grant “FIT—Fondo speciale rotativo per l’Innovazione Tecnologica,” E03/00815/00/x17, program title: “Innovative natural active ingredients for the cosmetic industry.”
References
- R Vyumvuhore et al, Effects of atmospheric relative humidity on stratum corneum structure at the molecular level: Ex vivo Raman spectroscopy analysis, Analyst 138 4103–4111 (2013)
- SW Youn, JI Na, SY Choi, CH Huh and KC Park, Regional and seasonal variations in facial sebum secretions: A proposal for the definition of combination skin type, Skin Res Tech 11 189–95 (2005)
- MD Cooper, H Jardine and J Ferguson, Seasonal influence on the occurrence of dry flaking facial skin, in R Marks and G Plewig, eds. The Environmental Threat to the Skin vol 159, Martin Dunitz, London (1992) pp 159–164
- J Rogers, C Harding, A Mayo, J Banks and A Rawlings, Stratum corneum lipids: The effect of aging and the seasons, Arch Dermatol Res 288 765–70 (1996)
- F Andersen, K Andersen and A Kligman, Xerotic skin of the elderly: A summer versus winter comparison based on biophysical measurements, Exog Dermatol 2 190–194 (2004)
- W Uter, O Gefeller and HJ Schwanitz, An epidemiological study of the influence of season (cold and dry air) on the occurrence of irritant skin changes of the hands, Br J Dermatol 138 266–272 (1998)
- AK Jha and D Gurung, Seasonal variation of skin diseases in Nepal: A hospital based annual study of outpatient visits, Nepal Med Coll J 8 266–268 (2006)
- A Callahan et al, Winter season, frequent handwashing and irritant patch test reactions to detergents are associated with hand dermatitis in health care workers, Dermatitis 24 170–175 (2013)
- HY Lee, M Stieger, N Yawalkar and M Kakeda, Cytokines and chemokines in irritant contact dermatitis, Mediators Inflamm ID 916497 (Nov 25, 2013)
- HR Smith, DA Basketter and JP McFadden, Irritant dermatitis, irritancy and its role in allergic contact dermatitis, Clin Exper Dermatol 27 138–146 (2002)
- S Lisby and O Baadsgaard, Mechanisms of irritant contact dermatitis, in Contact Dermatitis, PJ Frosch, T Menne and JP Lepoittevin, eds, Springer, Berlin (2006) pp 69–82
- AK Deodhar and RE Rana, Surgical physiology of wound healing: A review, J Postgrad Med 43 52–56 (1997)
- JM Johnsonm, CT Minson and DL Kellogg, Jr, Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation, Compr Physiol 4 33–89 (2014)
- N Charkoudian, Skin blood flow in adult human thermoregulation: How it works, when it does not, and why, Mayo Clin Proc 78 603–12 (2003)
- A Theocharis, C Clément and EA Barka, Physiological and molecular changes in plants grown at low temperatures, Planta 235 1091–105 (2012)
- C Lütz, Cell physiology of plants growing in cold environments, Protoplasma 244 53–73 (2010)
- WC Xu, JG Shen and JQ Jiang, Phytochemical and biological studies of the plants from the genus Daphne, Chem Biodivers 8 1215–33 (2011)
- F Cottiglia, G Loy, D Garau, C Floris, M Casu, R Pompei and L Bonsignore, Antimicrobial evaluation of coumarins and flavonoids from the stems of Daphne gnidium L, Phytomedicine 8 302–305 (2001)
- CH Hong, SK Hur, OJ Oh, SS Kim, KS Nam and SK Lee, Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells, J Ethnopharmacol 82 153–159 (2002)
- M Deiana, A Rosa, V Casu, F Cottiglia, L Bonsignore and MA Dessi, Chemical composition and antioxidant activity of extracts from Daphne gnidium L, J Amer Oil Chem Soc 80 65–70 (2003)
- E Kupeli, A Tosun and E Yesilada, Assessment of anti-inflammatory and antinociceptive activities of Daphne pontica L. (Thymelaeaceae), J Ethnopharmacol 113 332–337 (2007)
- W Zheng, X Gao, C Chen and R Tan, Total flavonoids of Daphne genkwa root significantly inhibit the growth and metastasis of Lewis lung carcinoma in C57BL6 mice, Intern Immunopharmacol 7 117–127 (2007)
- MY Lee et al, Anti-inflammatory activity of (−)-aptosimon isolated from Daphne genkwa in RAW264.7 cells, Intern Immunopharmacol 9 878–885 (2009)
- I Süntar et al, Efficacy of Daphne oleoides subsp. kurdica used for wound healing: Identification of active compounds through bioassay guided isolation technique, J Ethnopharmacol 141(3) 1058–70 (2012)
- C Couteau, LJ Coiffard and V Sébille-Rivain, Influence of excipients on moisturizing effect of urea, Drug Dev Ind Pharm 32(2) 239–42 (2006)
- U Heinrich et al, Multicenter comparison of skin hydration in terms of physical, physiological and product-dependent parameters by the capacitive method (Corneometer CM 825), Int J Cosmet Sci 25 45–53 (2003)
- GR Leonardi, LR Gaspar and PM Maia Campos, Application of a non-invasive method to study the moisturizing effect of formulations containing vitamins A or E or ceramide on human skin, J Cosmet Sci 53, 263–8 (2002)
- F Li, E Conroy, M Visscher and RR Wickett, The ability of electrical measurements to predict skin moisturization. I. Effects of NaCl and glycerin on short-term measurements, J Cosmet Sci 52 13–22 (2001)
- M Taniguchi and K Baba, Three bioflavanoids from Daphne odora, Phytochem 42 1447–1453 (1996)
- C Manach, A Scalbert, C Morand, C Rémésy and L Jiménez, Polyphenols: Food sources and bioavailability, Am J Clin Nutr 79 727–47 (2004)
- OV Zillich, U Schweiggert-Weisz, P Eisner and M Kerscher, Polyphenols as active ingredients for cosmetic products, Int J Cosmet Sci 37 455–64 (2015)
- RS Greene, DT Downing, PE Pochi and JS Strauss, Anatomical variation in the amount and composition of human skin surface lipid, J Invest Dermatol 54 240–247 (1970)
- FK Habib et al, The localization and expression of 5 alpha-reductase types I and II mRNAs in human hyperplastic prostate and in prostate primary cultures, J Endocrinol 156 509–17 (1998)
- JM Carthy, TGFb signaling and the control of myofibroblast differentiation: Implications for chronic inflammatory disorders, J Cell Physiol (2017) doi: 10.1002/jcp.25879
- KS Ha and JH Exton, Activation of actin polymerization by phosphatidic acid derived from phosphatidylcholine in IIC9 fibroblasts, J Cell Biol 123 1789–96 (1993)
- BA Hocevar, TL Brown and PH Howe, TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway, Embo J 18 1345–56 (1999)
- AD Bradshaw and EH Sage, SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury, J Clin Invest 107 1049–54 (2001)
- AD Bradshaw, The role of SPARC in extracellular matrix assembly, J Cell Commun Signal 3 239–46 (2009)
- GN Stamatas, AP Morello and DA Mays, Early inflammatory processes in the skin, Curr Mol Med 13 1250–69 (2013)
- D Wang et al, Synthetic LXR agonist T0901317 attenuates lipopolysaccharide-induced acute lung injury in rats, Int Immunopharmacol 11 2098–103 (2011)
- N Ferrara, The role of VEGF in the regulation of physiological and pathological angiogenesis, EXS 94 209–31 (2005)
- P Carmeliet, VEGF as a key mediator of angiogenesis in cancer, Oncology 69 suppl 3 4–10 (2005)
- L Shih-Chen, Chinese Medicinal Herbs: A Modern Edition of a Classic Sixteenth-century Manual, Dover Publications Inc, Mineola, NY (1973)
- S Liang et al, Bioflavonoids from Daphne feddei and their inhibitory activities against nitric oxide production, Chem Pharm Bull 56 1729–1731 (2008)
- E Yesilada et al, In vitro inhibitory effects of Daphne oleoides subsp. oleoides on inflammatory cytokines and activity-guided isolation of active constituents, Cytokine 13 359–364 (2009)
- S Takai, M Sakaguchi, J Denan, K Baba and M Miyazaki, Effects of daphnodorin A, daphnodorin B and daphnodorin C on human chymase-dependent angiotensin II formation, Life Sci 64 1889–96 (1999)
- DJ Campbell, Do intravenous and subcutaneous angiotensin II increase blood pressure by different mechanisms? Clin Exp Pharmacol Physiol 40 560–70 (2013)
- M Antunes-Ricardo, JA Gutiérrez-Uribe, C Martínez-Vitela and SO Serna-Saldívar, Topical anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica, Biomed Res Int 847320 (2015) doi: 10.1155/2015/847320
- CF Lin, YL Leu, SA Al-Suwayeh, MC Ku and TL Hwang and JY Fang, Anti-inflammatory activity and percutaneous absorption of quercetin and its polymethoxylated compound and glycosides: The relationships to chemical structures, Eur J Pharm Sci 47 857–64 (2012)
- D Fratantonio, A Speciale, D Ferrari, M Cristani, A Saija and F Cimino, Palmitate-induced endothelial dysfunction is attenuated by cyanidin-3-O-glucoside through modulation of Nrf2/Bach1 and NF-kB pathways, Toxicol Lett 239 152–60 (2015)
- M Yamamoto et al, Hesperidin metabolite hesperetin-7-O-glucuronide, but not hesperetin-3'-O-glucuronide, exerts hypotensive, vasodilatory and anti-inflammatory activities, Food Funct 4 1346–51 (2013)
- A Barbulova, F Apone and G Colucci, Plant cell cultures as source of cosmetic active ingredients, Cosmetics 1 94–104 (2014)
- M Bimonte, A Tito, A Carola, A Barbulova, J Hill, M Cucchiara, F Apone and G Colucci, Dolichos cell culture extract for protection against UV damage, Cosm & Toil 129 46–56 (2014)
- A Tito, M Bimonte, A Carola, A De Lucia, A Barbulova, A Tortora, G Colucci and F Apone, An oil-soluble extract of Rubus idaeus cells enhances hydration and water homeostasis in skin cells, Int J Cosmet Sci 37 588–594 (2015)
- EK Lee et al, Cultured cambial meristematic cells as a source of plant natural products, Nat Biotechnol 28 1213–7 (2010)
- A Tito at al, A tomato stem cell extract containing antioxidant compounds and metal chelating factors protects skin cells from heavy metal induced damages, Int J Cosmet Sci 33 543–552 (2011)