Read the full article in the January 2021 digital edition. . .
The paths of two different fields of research have begun to converge: the study of the skin microbiome, and bacteriophages as an alternative to antibiotics to combat bacterial pathogens. Several bacteria are natural residents on the human skin, some of which contribute to its health and others that can have deleterious effects. It is often when an imbalance in species occurs that undesirable skin conditions follow.
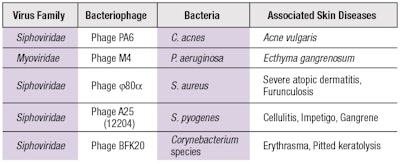
Naturally occurring bacteriophages that uniquely target specific bacterial species (see Table 1) present a tool that could be applied to modulate the growth of certain populations of bacteria—i.e., so-called phage therapy. For instance, Cutibacterium acnes (formerly Propionibacterium acnes) is understood to contribute to Acne vulgaris. A cluster of bacteriophages exists that only attacks and replicates inside of this particular bacterial species, which culminates in the destruction of the bacteria. Therefore, a topical formulation containing bacteriophages against C. acnes could represent a new skin care agent to combat acne. Similarly, this approach could be replicated with many other resident microbes on the skin that are responsible for a number of skin diseases and conditions.
This article offers a commentary in support of bacteriophages, or phages, in skin care therapeutics. It provides their brief evolutionary history, considers their utility as antimicrobial alternatives, and posits their application in modern skin care.
Bacteriophages
Viruses that target bacteria are collectively called bacteriophages and represent the largest domain of life on Earth. These microorganisms are ancient and have kept numerous bacterial species in check probably since the emergence of microbial life.
For reasons yet to be fully understood, as life evolved, viruses targeting multicellular organisms developed ribonucleic acid (RNA)-based genomes with cylindrical and icosahedral shaped structures. In contrast, viruses that attack bacteria predominantly have deoxyribonucleic acid (DNA)-based genomes with structures that resemble the lunar lander modules (see Figure 1).
This schism in the shape and genetic content of viruses reinforces why bacteriophages are uniquely safe as potential antibacterial therapeutics: millions of years of evolution have made them so significantly different from multicellular life that they can no longer present a health threat to humans, animals or plants—but they are very much a predator to bacterial life.1, 2
History and Production
In the time before DNA and RNA had been characterized, and the first viruses were identified, scientists experimented with filterable substances that appeared to have a beneficial impact on people afflicted with certain bacterial diseases. The term filterable substances was used to refer to disease-causing agents that could not be filtered from solutions due to their extremely small size—unlike bacteria that could be removed from solutions via 0.2-micron porcelain filters. This approach allowed Russian biologist Dmitri Ivanovsky to experimentally demonstrate in 1892 that there were microscopic pathogens much smaller than bacteria. As it turns out, many of the agents found in these substances were in fact viruses.3
One of the early pioneers who used viruses to replicate inside of bacteria as antimicrobials was the French-Canadian scientist Felix d’Herelle, Ph.D. Indeed, he was one of the co-discoverers of bacteriophages in 1917 and did much to advance the field of applied microbiology with his experiments into what has become known as phage therapy.4
d’Herelle developed a procedure for the propagation and purification of bacteriophages, the fundamentals of which are still practiced today. First, nutrient-rich media is used to cultivate the bacterial species of interest, whose growth causes the media to become turbid.
Subsequently, the bacterial culture is inoculated with bacteriophages known to target and replicate inside of that particular species of bacteria. As the bacteriophages replicate through their lytic cycle, the bacteria are destroyed and the turbid media becomes translucent. Finally, the liquid media is filtered to retain the resulting bacterial debris but not the filterable bacteriophages.
Upon the discovery of penicillin and other antibiotics, phage therapy was largely abandoned in the Western world. However, it became the antimicrobial agent of choice for the former Soviet Union, where it continues to be employed to this day. With the rising threat of antibiotic-resistant bacteria, the Western world has taken a fresh look at phage therapy as an alternative to antibiotics.
Phage Therapy Today
Enter the modern era, with a global catastrophe brewing thanks to the advent and increasing incidence of antibiotic-resistant bacteria.5-7 Multiple classes of antibiotics are proving ineffective in treating bacterial pathogens that were once nullified as a public health threat, and the pace of antibiotic research and development has slowed to a crawl.
. . .Read more in the January 2021 digital edition. . .
References
- Gebru, E., Lee, ... Park, S.C., et al. (2010). Effect of probiotic-, bacteriophage- or organic acid-supplemented feeds or fermented soybean meal on the growth performance, acute-phase response and bacterial shedding of grower pigs challenged with Salmonella enterica serotype Typhimurium. J Anim Sci 88 3880-6.
- Jamal, M., Bukhari, S., ... Shah, S.S.A., et al. (2019). Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J Basic Microbiol 59 123-133.
- Bridger, J.C. (2008). From filterable agents to virus genes: The discovery of viruses that cause diarrhoea. Vet J 178 5-6.
- Abedon, S.T., Kuhl, S.J., Blasdel, B.G. and Kutter, E.M. (2011). Phage treatment of human infections. Bacteriophage 1 66-85.
- Aslam, B., Wang, W., ... Baloch, Z., et al. (2018). Antibiotic resistance: A rundown of a global crisis. Infect Drug Resist 11 1645-1658.
- Betts, J.W., Hornsey, M. and La Ragione, R.M. (2018). Novel antibacterials: Alternatives to traditional antibiotics. Adv Microb Physiol 73 123-169.
- Medina, E. and Pieper, D.H. (2016). Tackling threats and future problems of multidrug-resistant bacteria. Curr Top Microbiol Immunol 398 3-33.
a The DermaPhage line of skin care by Biocogent LLC is currently under development.