This column concludes a three-part series on techniques for epidermal-dermal separation (see Figure 1). It focuses on heat and mechanical techniques and describes the advantages and disadvantages of each. Previous installments reviewed enzyme digestion options and the use of acid, alkalis and natural salts.
Heat Separation
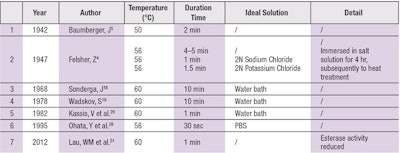
Epidermal-dermal separation by heat (see Table 3) was introduced in 1942 by Baumberger et al. Since increasing temperatures can soften collagen fibers, temperatures much lower than the boiling point of water may be used to separate the epidermis from the dermis.
For example, after exposure for 2 min on a slide warming table at 50°C, the epidermis was found to peel readily and completely using forceps. Complete division occurred with ease at 49.2°C, after incompleteness at 48.2°C. With temperatures above 51°C, detachment difficulty occurred. Felsher4 detected an influence of salts on the subsequent separation of the epidermis by heat. Heat separation is facilitated by ions, which can promote collagen swelling or depression.
In the studies of Sonderga et al.18 and Wadskov et al.,19 separation of the epidermis from the dermis in normal human skin by heat was reported possible after 10 min at 60°C.
Kassis et al.20 analyzed epidermal and dermal prostaglandins by heat separation. They kept frozen biopsy specimens in a water bath for various times and found that heating at 60°C for 1 min produced a distinct separation of epidermis from dermis. As there was no significant change in Prostaglandin E1 (PGE1) activity after 1 min of heating, this heat separation technique seemed a reliable and quick method for PG analysis of skin.
Esterases are important for topical or transdermal delivery of drugs since these enzymes may catalyze the hydrolysis of pharmaceuticals containing ester bonds. Lau et al.21 detected esterase activity in fresh and heat-separated porcine ear skin. Esterase activity was visibly diminished in common heating separated skin (compared to fresh skin). After heating at 60°C for 1 min, neither epidermis nor the stratum corneum exhibited positive histochemical staining for esterases activity. Therefore, heat separation should not be used to study the permeation of ester-containing permeants.
Mechanical Methods
The methods above have the disadvantage of altering, to some degree, the physical and/or chemical integrity of one or both layers and may therefore be unsatisfactory for studies that require an isolated—but otherwise intact—epidermis or dermis. There is a need for techniques by which epidermis can be separated from the dermis via purely mechanical forces, to avoid chemical or thermal damage.
Stretching
Van Scott22 stretched the skin and removed the epidermis as a continuous sheet by means of a razor blade or scalpel. When skin is stretched, the epidermis slips off easily using this procedure and can be rapidly removed. Gilbert23 and Sputt24 improved the technique so skin only needed stretching to a relaxed length. However, these methods require comparatively large surgical necropsy specimens, and when scraped off with a scalpel, may lack a basal cell layer.
Suction
As with unidirectional stretching, dermal-epidermal separation has been achieved by the application of pressure gradients causing multidirectional distension. In 1878, Unna25 described the separation of the epidermis from the dermis along the dermal-epidermal junction (DEJ) during dry cupping. Blank et al.26 also separated the epidermis from the dermis with vacuum via bullae formation, which demonstrated DEJ separation histologically. This approach therefore became a mechanical method to separate the epidermis. In vivo separation of the complete human epidermis by suction was accomplished in 1964.
Kiistala et al.27 found that suction at a pressure of 20 cm Hg applied to intact human healthy skin produced blisters within 2 hr, the roof of which consisted solely of epidermis and included the basal cell layer. They constructed a device that produced standard suction blisters of preselected size and number on human skin without discomfort. In approx. 2 hr, suction at 150 mm Hg28 permitted separation of the epidermis from cadavers and certain animals. The blister roof consisted of viable, full-thickness epidermis and the clear fluid was a non-inflammatory transudate.
No scarring resulted and the suction trauma of the dermis was minimal. Thus, the suction epidermis appeared useful for tissue culture and biochemical analyses. Today, this method is widely applied in dermatological research and practice for epidermal grafting29–31 for creating standardized wound-healing models32, 33 and for studying morphological, physiological or pharmacological phenomena.34, 35
There is need for techniques by which the epidermis can be separated from the dermis via purely mechanical forces, to avoid chemical or thermal damage.
Overall Pros and Cons
Many techniques relying on different mechanisms have been described in this series.
Heat is simple, yet tedious. Although the heat process has been used successfully, chemical reagents are effective but disturb the electrolyte cellular equilibrium. Digestion by enzymes gives complete separation but it destroys important components. Meanwhile, mechanical division necessitates a relatively large tissue piece but has the advantage in that chemical changes do not occur. Many investigations have compared these methods and documented their different influences on the structure and function of skin biochemistry.
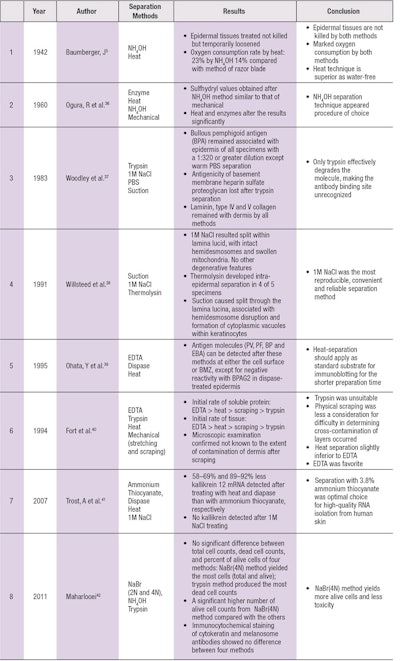
Baumberger, when comparing NH4OH and heat separation methods, found that epidermal tissues treated were not killed but only temporarily loosened, and there was a marked decrease in oxygen consumption by the epidermis by both methods. However, the heat technique may be superior because the tissue is not brought into water and there is no loss of material (see Table 4).
Quantitative determinations of stratum corneum sulfhydryl and disulfide concentration revealed relatively consistent values in certain skin diseases. Various methods of separation of epidermal sulfhydryl showed its values obtained after ammonium separation resembled those following mechanical separation, while heat and enzymes appeared to alter the results significantly and were deemed not satisfactory.36
Dermo-epidermal separation is an essential technique for immunoblotting studies and for the diagnostic immunofluorescence of autoimmune bullous diseases. To study the localization of basement membrane components after different separation methods, Woodley et al.37 separated adult human skin using four methods. Only the basement membrane heparan sulfate proteoglycan was trypsin-sensitive.
Willsteed et al.38 examined the electron microscopic appearance of specimens separated by three methods, concluding that 1M NaCl remains the most convenient and reproducible means of inducing dermal-epidermal separation through the lamina lucida.
Ohata et al.39 compared the autoantigens for bullous skin diseases by immunoblotting using different dermal-epidermal separation techniques; the results suggest that EDTA-, dispase- and heat-separated skin were similar for detecting various autoantigens but heat separation is preferable because the preparation time is shorter.
To compare the effects of epidermal-dermal separation techniques of hairless mouse skin, the most suitable method was determined by kinetic studies in homogenate medium.40 Based on data of the effects of separation methods on soluble protein yield and dermal enzymatic activity using DBMTX as a substrate, EDTA treatment appears a favorable method for epidermal separation.
To obtain rapid, high-quality epidermal-specific RNA from human skin, Trost et al.41 investigated the effect of dermal-epidermal separation methods—including ammonium thiocyanate, dispase, heat and 1M NaCl—and concluded that the fast chemical separation method with 3.8% ammonium thiocyanate is the optimal choice for producing high-quality RNA isolation from human skin.
Epidermis separation is also useful in autologous skin transplantation. The first step in isolating epidermal cells is to separate it from dermis. Recently, several methods for epidermis isolation and epidermal cell suspension were compared to select the optimum one for clinical use. NaBr (4N) method is considered as the least toxic and the most viable cells produced.42
Conclusion
In conclusion, the separation method must be appropriate for the experimental project, and no single method appears superior for all purposes. Future comparative investigation should benefit the integrity of skin care, and cosmetic research based on this methodology and predictability will lead to greater utilization, as more refined knowledge should lead to enhanced research integrity.
References
- Z Felsher, Studies on the adherence of the epidermis to the corium, J Invest Dermatol 8 35-47 (1947)
- J Søndergaard, H Zachariae, Epidermal histamine, Arch Klin Exp Dermatol, 233 323–328 (1968)
- S Wadskov, J Søndergaard, Determination of cyclic-amp in heat-separated human epidermal tissue, Acta Derm Venereol, 58 191–195 (1978)
- V Kassis, J Søndergaard, Heat-separation of normal human-skin for epidermal and dermal prostaglandin analysis, Arch Dermatol Res, 273 301–306 (1982)
- WM Lau, KW Ng, K Sakenyte, CM Heard, Distribution of esterase activity in porcine ear skin, and the effects of freezing and heat separation, Int J Pharm 433 10–15 (2012)
- EJ Van Scott, Mechanical separation of the epidermis from the corium, J Invest Dermatol 18 377–379 (1952)
- D Gilbert, PD Mier, TE Jones, An improved technic for the isolation of epidermis from human skin, J Invest Dermatol 40 165–167 (1963)
- D Sprutt, An improved technic for the isolation of epidermis from larger specimens of human skin, J Invest Dermatol 42 285 (1964)
- P Unna, Zur Anatomie der Blasenbildung an der menschlichenHaut, Vjschr Dermatol Syphil 5 3 (1878)
- IH Blank, OG Miller, A method for the separation of the epidermis from the dermis, J Invest Dermatol 15 9–10 (1950)
- U Kiistala, KK Mustakallio, In-vivo separation of epidermis by production of suction blisters, Lancet 7348 1444–1445 (1964)
- U Kiistala, Suction blister device for separation of viable epidermis from dermis, J Invest Dermatol 50 129–137 (1968)
- S Gupta, S Shroff, Modified technique of suction blistering for epidermal grafting in vitiligo, Int J Dermatol 38 306–309 (1999)
- R Falabella, Suction blister device for separation of viable epidermis from dermis, Clin Exp Dermatol 29 105–106 (2004)
- J Li, WW Fu, ZZ Zheng, QQ Zhang, Y Xu, L Fang, Suction blister epidermal grafting using a modified suction method in the treatment of stable vitiligo: a retrospective study, Dermatol Surg 37 999–1006 (2011)
- J Kottner, K Hillmann, S Fimmel, S Seite, U Blume-Peytavi, Characterisation of epidermal regeneration in vivo: a 60-day follow-up study, J Wound Care 22 395–400 (2013)
- V Czaika, A Alborova, H Richter et al., Comparison of transepidermal water loss and laser scanning microscopy measurements to assess their value in the characterization of cutaneous barrier defects, Skin Pharmacol Physiol. 25 39–46 (2012)
- JJ Levy, J Vonrosen, J Gassmuller, RK Kuhlmann, L Lange, Validation of an in-vivo wound-healing model for the quantification of pharmacological effects on epidermal regeneration, Dermatology 190 136–141 (1995)
- IG Panoutsopoulou, G Wendelschafer-Crabb, JS Hodges, WR Kennedy, Skin blister and skin biopsy to quantify epidermal nerves A comparative study, Neurology 72 1205–1210 (2009)
- R Ogura, JM Knox, AC Griffin, Separation of epidermis for the study of epidermal sulfhydryl, J Invest Dermatol 35 239–243 (1960)
- D Woodley, D Sauder, MJ Talley, M Silver, G Grotendorst, E Qwarnstrom, Localization of Basement-Membrane Components after Dermal-Epidermal Junction Separation, J Invest Dermatol 81 149–153 (1983)
- EM Willsteed, BS Bhogal, AB Das et al., An Ultrastructural Comparison of Dermo-Epidermal Separation Techniques, J Cutan Pathol 18 8–12 (1991)
- Y Ohata, T Hashimoto, T Nishikawa, Comparative study of autoantigens for various bullous skin diseases by immunoblotting using different dermo-epidermal separation techniques, Clin Exp Dermatol 20 454–458 (1995)
- JJ Fort, AK Mitra, Effects of epidermal/dermal separation methods and ester chain configuration on the bioconversion of a homologous series of methotrexate dialkyl esters in dermal and epidermal homogenates of hairless mouse skin, Int J Pharm 102 241–247 (1994)
- A Trost, JW Bauer, C Lanschuetzer et al., Rapid, high-quality and epidermal-specific isolation of RNA from human skin, Exp Dermatol 16 185–190 (2007)
- MK Maharlooei, AA Mohammadi, A Farsi, I Ahrari, A Attar, A Monabati, A comparison between different existing methods used to separate epidermal cells from skin biopsies for autologous transplantation, Indian J Dermatol 56 666–669 (2011)