This article describes tools such as organic conceptual diagrams, Hansen solubility parameters and alcohol classes to study polyol dynamics for smarter makeup remover design. Effects on CMC, cloud point and other functional benefits are also considered.
For many consumers, makeup has been integrated into daily work and social interactions, in turn making its removal a daily task to maintain skin health. According to projections,1 the market for makeup removers is estimated to reach US $805.2 million by 2031, expanding at a CAGR of 4.1% from 2024 to 2031.1
With the application of new raw materials and technologies in makeup, the requirements for makeup removal has increased accordingly. This has extended traditional makeup removers, such as cleansing oils, milks and eye and lip makeup removers, into new forms such as cleansing balms, creams and gels.
Cleansing oils face the difficulty of frequently updated water-soluble film-forming agents, giving rise to bi-continuous phase makeup remover liquidsa. At the same time, the balm makeup remover category has become relatively popular, which solves the problem of cleansing oil’s low viscosity and dripping during use.
From a formulating perspective, the application of polyols in makeup removal products is gradually increasing. As such, the present article compares the characteristics and advantages of various polyols using existing technical theories and data to aid formulators in choosing poloyls or other materials to advance makeup remover formula designs. Specifically described are the use of tools such as organic conceptual diagrams, Hansen solubility parameters and alcohol classifications theory.
The impact of polyols on critical micelle concentration (CMC) and cloud point of nonionic surfactants are also important considerations, the dynamics of which can be explained by these tools, as will be shown. Finally, the moisturizing effects, antimicrobial synergies, safety and sensory properties of polyols are examined, to round out the makeup remover formulation.
Organic Conceptual Diagram and Inorganic-organic Balance (IOB)
The first tool to consider is an organic conceptual diagram. Here, polyols are visualized on a graph based on their inorganic vs. organic values. Under the principles of the organic conceptual diagram, properties that depend largely on van der Waals forces are referred to as organic properties, while those that depend largely on electron affinity are called inorganic properties. The ratio of inorganic to organic values (IV/OV) is referred to as the inorganic-organic balance (IOB).2
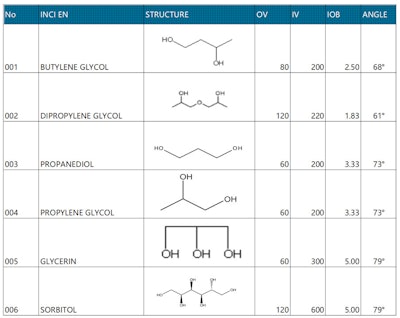
Table 1 (see below) lists the IOB values and emulsification angles of common polyols including butylene glycol, dipropylene glycol, propylene glycol, propanediol, glycerin and sorbitol.2 Figure 1 (see below) plots their organic and inorganic information in an organic conceptual diagram.3
The IOB value can be used as a numeric reference index to determine the polyol’s balance of hydrophilicity and lipophilicity; note that subtle differences in structure also affect the properties of matter but these are not discussed in depth in the present article. While traditionally, product developers have used the HLB value of the emulsifier as the main reference to design formulas, the IOB value can provide a more intuitive reference number for the hydrophilicity and lipophilicity of polyols, oils, etc., that are not part of the surfactant (emulsifier) – as will be shown.
From the perspective of hydrophilicity and lipophilicity, dipropylene glycol (DPG), propanediol (PDO) and butylene glycol (BG) are more lipophilic; glycerin and sorbitol are more hydrophilic; and propylene glycol (PG) as well as PDO are in the middle. From the perspective of molecular weight (distance from the origin in Figure 1), PDO, BG and PG are shorter, while glycerin, DPG and sorbitol are longer. These characteristics impact various other formula dynamics, described here.
Hansen Solubility Parameters of Polyols
Hansen solubility parameters (HSPs) are another useful tool to determine the affinity or compatibility of two materials for one other. HSPs can approximate the theoretical solubility of a solvent to dissolve a substance under certain conditions, which aids in the comparison of chemical substances for formula design. HSPs calculate the distance between substances using three parameters:4
- δD dispersion force: The energy of intermolecular dispersion forces;
- δP polar force:The energy of intermolecular dipole forces; and
- δH hydrogen bonding: The energy of intermolecular hydrogen bonding forces.
For example, consider the following calculation for two molecules: Molecule 1 [δD1, δP1, δH1] and Molecule 2 [δD2, δP2, δH2]:
Distance D2 = 4(δD1-δD2)2+(δP1-δP2)2+(δH1-δH2)2
When D < 4, the two molecules are miscible; when D > 8, the two molecules are insoluble; and there is an intermediate zone where they are partially or slightly dissolved in one another; note that the radius of the material sphere must be considered.
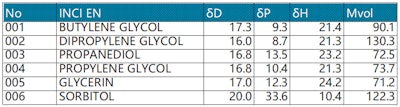
HSPs of common polyols: Table 2 (see below) shows the HSP parameter values of the common polyols butylene glycol, dipropylene glycol, propylene glycol, propanediol, glycerin and sorbitol. These values can be used as supplements or evidence for the IOB to further analyze differences or relationships in properties caused by different intermolecular forces as a prediction and reference for designing formulas.
For example, from the perspective of the IOB value, propanediol and propylene glycol are the same (3.33), but from the perspective of HSPs, they are different in terms of δP polar force and δH hydrogen bonding adhesion force. This difference could impact safety or other aspects of the materials. In addition, the HSPs can be used as a reference prediction to calculate the distance of different polyols to dissolve the target water-soluble film-forming agent or polymer material in makeup.
Alcohol Classifications and Water Solubility
Alcohol classifications are yet another way to characterize materials. Consider an alcohol is an organic compound with at least one hydroxyl functional group (OH) bound to a saturated carbon atom. Alcohols can be classified as primary, secondary or tertiary depending on the number of alkyl substituents attached to the carbon bonded to the O-H group.
In a primary alcohol, the carbon that carries the -OH group is only attached to one alkyl group. In a secondary alcohol, the carbon to which the -OH group is attached is joined directly to two alkyl groups that may be the same or different. In a tertiary alcohol, the carbon atom holding the -OH group is attached directly to three alkyl groups, which may be any combination of the same or different groups (see Figure 2, below).5
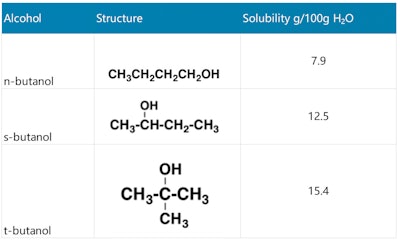
The solubility of isomeric alcohols increases with branching because the surface area of the hydrocarbon part decreases with branching. Thus: water solubility = primary alcohol < secondary alcohol < tertiary alcohol. The water solubility of different butanol structures is shown in Table 3 (see below).
Since the organic conceptual diagram theory cannot distinguish the position of the hydroxyl group of polyols on the carbon chain resulting in differences in water solubility, the alcohol classifications theory of polyols is useful as a supplement.
Polyols vs. CMC of Nonionic Surfactants
The way the CMC of surfactants in water changes with the additional of polyols is an important consideration for makeup removers (see Figure 3).6 As can be seen, as the amount of butylene glycol and propylene glycol added increases, the CMC of the surfactant shows an upward trend. As the amount of glycerin added increases, the CMC of the surfactant decreases initially but with further addition, the CMC of the surfactant increases again. With sorbitol, however, as the amount added increases, the CMC of the surfactant shows a downward trend.
Organic conceptual design and HSPs: For the present analysis, the organic conceptual diagram and HSPs can explain the influence of polyols on surfactant CMC. First of all, the hydrogen bonding between polyols and nonionic surfactants is affected by the hydrophilicity and lipophilicity of the polyol itself (from the perspective of IOB) and polar force (from the perspective of HSP δP dipole moment). The smaller the IOB, the deeper it is inserted into the micelle, and the closer to the lipophilic group. The larger δP is, the closer it is to the micelle surface and closer to the water phase.
On the other hand, the hydrogen bonding ability of the polyol and the hydrophilic group of the surfactant will also be strong or weak due to the difference in the δH. Refer to Table 1 for the IOB and location of organic conceptual diagram of polyols, as well as the IOB of 1.11 (IV 495, OV 480) and the location of organic conceptual diagram of polyoxyethylene (6) dodecyl ether shown in Figure 4, below.
Ratio line principle: According to the ratio line principle3 (see Figure 5, below) of the organic conceptual diagram, the relationship between butylene glycol, propylene glycol and polyoxyethylene (6) dodecyl ether may be in the soluble region. The relationship between glycerin, sorbitol and polyoxyethylene (6) lauryl ether may be in the slightly soluble zone or the soluble zone.
IOB value: From the IOB value, it can be judged that butylene glycol is the most lipophilic, followed by propanediol, glycerin and sorbitol, which are more hydrophilic. Since butylene glycol and propylene glycol are both hydrophilic and lipophilic, one may speculate they will be miscible with part of the surfactant. It is bound to the hydrogen bond of the hydrophilic group of the surfactant molecule but butylene glycol’s position is deeper into the micelle, closer to the lipophilic group, forming a mixed micelle, thereby widening the distance between surfactant molecules. This is the reason it increases the CMC of the surfactant.
The influence of the addition of polyol on the spacing of lamellar liquid crystal planes has been experimentally verified.6 According to the IOB value, it can be inferred that because butylene glycol is more lipophilic than propylene glycol, it can enter more deeply into the hydrophilic group of the surfactant molecule that forms the micelle, and butylene glycol is closer to the lipophilic group than propylene glycol. The ability to widen the distance between surfactant molecules will be stronger, so the ability to increase the surfactant CMC will also be stronger.
Glycerin and sorbitol: Glycerin and sorbitol may be in the soluble or slightly soluble region, so they are more likely to bind via hydrogen bonding to the hydrophilic groups of the surfactant molecules in the shallower parts of the micelles (the hydrophilic groups of the surfactant molecules closer to water phase part). This reveals that while the IOB values of glycerin and sorbitol are the same, their effects on CMC are different, so the HSP must be introduced for further analysis.
In doing such, it was found the δH hydrogen bonding force of sorbitol was much smaller than glycerin, but the δP dipole moment force was much greater than that of glycerin. It can be speculated that both glycerin and sorbitol are located where the hydrophilic group of the surfactant is closer to the water phase at low concentrations. However, the δP of sorbitol is greater than glycerin, so it is closer to the hydrophilic side. It has a stronger ability to open the distance between hydrophilic groups and reduces CMC more significantly.
As the concentration increases, more glycerin accumulates in the shallow parts of the hydrophilic groups of the surfactants forming micelles, causing an increase in the distance between the internal glycerin and the external water. Because the glycerin δP is smaller and δH is greater, it is squeezed inward to bind to the hydrogen bonds closer to the depth of the hydrophilic group of the surfactant forming micelles.
This causes an effect similar to diol’s, widening the distance between surfactant molecules and increasing the CMC of the surfactant. When the concentration increases further and the distance between the surfactant molecules reaches the gravitational limit of the lipophilic group, micelles can no longer be formed and the CMC curve stops (with the curve close to 0, micelles no longer form).
As the concentration of sorbitol increases, it also accumulates in the shallow place of the hydrophilic groups of the surfactant of the micelle. Sorbitol has a greater δP and smaller δH, therefore it cannot enter into the depth of the hydrophilic group of the surfactant of the micelle, so it continues to expand at the shallower position of the hydrophilic group of the micelle, causing the CMC to continue to decrease. When the concentration increases further and the number of polymerized surfactant molecules drops to a specified threshold, micelles can no longer be formed and the CMC curve stops.
In addition, although the IOB values of glycerin and sorbitol are the same, there are differences in their hydrophilicity. According to the theory of alcohol classification and water-solubility previously described, glycerin has two primary hydroxyl groups and one secondary hydroxyl group, and sorbitol has two primary hydroxyl groups and four secondary hydroxyl groups. Secondary hydroxyl groups are more hydrophilic than primary hydroxyl groups. Therefore, sorbitol is more hydrophilic than glycerin, which also explains why sorbitol cannot enter into the micelle.
Propanediol and propylene glycol: In analyzing the impact of propanediol on CMC, although the IOB values of propanediol and propylene glycol are the same, there are still some differences that could have an impact. Considering the alcohol classifications theory, propanediol contains two primary hydroxyl groups whereas propylene glycol contains one primary hydroxyl group and one secondary hydroxyl group. This would make propylene glycol more hydrophilic than propanediol.
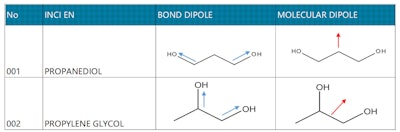
In addition, according to the theory of polar functional groups in organic chemistry, a hydroxyl (alcohol group) is an –OH group covalently bonded to a carbon atom. The oxygen atom is much more electronegative than either the hydrogen or the carbon, which will cause the electrons in the covalent bonds to spend more time around the oxygen than around the C or H.7 The dipole moment migrates in the direction of -O- (see Figure 6, below). When the two hydroxyl groups are closer, the electronegativity tendency is increased. Through this synergistic effect, the hydrogen bonding ability with water is enhanced, which means propylene glycol is more hydrophilic than propanediol.
In addition, the two hydroxyl groups of propylene glycol are biased to one side, while the two hydroxyl groups of propanediol are symmetrically distributed at both ends (see Table 4, below). In this way, the exposed carbon chain (methyl group) on propylene glycol is more lipophilic, and the methyl group is a non-polar functional group,7 which can easier insert deep into the micelle and closer to the lipophilic group, thus widening the distance between surfactant molecules.
Therefore, combining these two theories, propylene glycol is both more hydrophilic and lipophilic than propanediol. According to the next section, it can also be inferred that propanediol is less effective than propylene glycol in raising the cloud point, which means surfactants are less soluble in propanediol solutions than in propylene glycol solutions. In summary, then, propanediol can increase surfactant CMC as the concentration increases but its improvement capability is less than that of propylene glycol.
Polyols vs. Cloud Point of Nonionic Surfactants
The influence and mechanism of polyols on the cloud point of nonionic surfactants was also explored (see Figure 7, below).6 Consider how, as the temperature of the aqueous solution of nonionic surfactants increases, it becomes difficult to expose the oxygen atoms of the hydrophilic group, the hydrogen bonding force weakens, the surfactant molecules continue to aggregate to form infinite associations, and the water solubility continues to decrease. Eventually, precipitation and turbidity occur. In the present work, increasing the amounts of butylene glycol or propylene glycol were found to raise the cloud point of the surfactant, whereas increasing the amounts of glycerin or sorbitol lowered the cloud point of the surfactant.
To analyze these effects, one can again turn to the organic conceptual diagram and HSPs. Going back to Table 1, the IOB of polyoxyethylene (10) oleanol ether was calculated as 1.10 (IV 797, OV 760) and its location was plotted on the organic conceptual diagram (see Figure 8 below) as described previously.
Butylene and propylene glycols: Butylene glycol and propylene glycol have lower IOBs and will therefore be miscible with part of the surfactants, integrating with the hydrogen bonds in the deep hydrophilic groups (i.e., close to the lipophilic groups) of the nonionic surfactant molecule micelles. Mixed micelles are then formed, which widens the distance between nonionic surfactant molecules, preventing them from aggregating at high temperatures and improving the water solubility of nonionic surfactants.6
Since butylene glycol has a lower IOB value than propylene glycol, it can form hydrogen bonds in a deeper region of the hydrophilic group of the surfactant. It also has a greater ability to separate surfactant molecules. Therefore, butylene glycol contributes more to raising the cloud point. When the concentration increases to a certain level, the nonionic surfactant molecules are almost completely separated and their water solubility is no longer affected by temperature (< 100˚C), causing the cloud point to disappear within 100˚C.
Glycerin and sorbitol: As discussed previously, glycerin and sorbitol form hydrogen bonds at the shallower hydrophilic groups of the nonionic surfactant micelles. At this point, they block the hydrogen bonding ability between the surfactant and water, resulting in decreased water solubility and cloud points.
Although the IOB values of glycerin and sorbitol are the same, the influence on the cloud points are different. The reason is because the δP of sorbitol is greater and its δH is smaller, therefore sorbitol only exists in the shallower hydrophilic groups of the surfactant micelles.
The binding ability of the surfactant to water thus becomes smaller, and the hydrogen bonding ability with the surfactant become weaker, resulting in more sorbitol aggregation in the shallower region between surfactant molecules, and causing reduced water solubility and a lower cloud point.
In addition, as previously explained, compared with glycerin, sorbitol is more hydrophilic so it is drawn to water. It also hinders the hydrogen bonding ability of surfactant and water more than glycerin does, so the cloud point decreases more.
Propanediol and propylene glycol: In analyzing the impact of propanediol on cloud point, consider how, as previously mentioned, from the perspective of the direction of the dipole moment, propylene glycol has a greater deviation in the dipole moment than propanediol. Propylene glycol is also more easily inserted into the depths of micelles. So in summary, propanediol can also increase the cloud point as the concentration increase, but the ability is weaker than that of propylene glycol.
Their impacts on cloud point also differ because although propanediol and propylene glycol have the same IOB value, as previously described, propylene glycol is more hydrophilic and lipophilic than propanediol. The two hydroxyl groups of propylene glycol are biased to one side. This exposes the carbon chain (methyl group) on the other side, which can more easily insert deeply into the micelle and closer to the lipophilic group, preventing the hydrophilic groups of the surfactant from intertwining with each other.
Also, according to the alcohol classifications theory, propylene glycol is more hydrophilic than propanediol, which increases the solubility of the surfactant in water. These two factors combine to raise the cloud point of surfactants. Research in the literature has also shown that 1,3-butanediol has a stronger ability to raise the cloud point than 1,4-butanediol (see Figure 9, below).8
This effect can additionally be explained based on the bias of the dipole moment. The more the hydroxyl group is biased to one side, the greater the bias of the dipole moment, which makes it easier for the exposed carbon chain to be inserted deeply into the micelle. This, in turn, increases the ability to raise the cloud point; thus: 1,2-butanediol > 1,3-butanediol > 1,4-butanediol. Inferring from the above, propanediol can also rise the cloud point of the surfactant as the addition amount increases; but the improvement efficiency is lower than that of propylene glycol.
Additional Properties: Moisturizing Benefits
To compare the moisturizing benefits of various polyols, a 10% aqueous solution of polyol or purified water (control) was applied to the inner forearm of the human body at 5 μL/cm2. Polyols tested included: glycerin, propanediol, propanediol (5%) + glycerin (5%), propylene glycol and butylene glycol. Stratum corneum conductance was measured before application and 5 min to 60 min after application, where the initial conductance was set to 1 to determine the rate of change with time after product use (see Figure 10, below).9
Results showed that among the polyols, 10% propanediol significantly increased the rate of conductance, indicating improved moisture content in the stratum corneum, immediately after application. However, this effect was even greater when combined with glycerin – almost twice that of the other polyols.
According HSPs, the δP of propanediol is higher than that of the other polyols (except sorbitol) and its δH is also the second highest after glycerin. In general, its HSPs are closer to that of water, which may impart a moisturizing effect that could benefit makeup removal products.
Additional Properties: Antimicrobial Effects
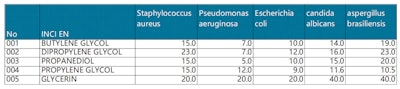
Common polyols were also compared in terms of their minimum inhibitory concentration (MIC) against microbes (see Table 5, below). The antibacterial activity of propanediol against various microorganisms was more potent than that of glycerin and dipropylene glycol; slightly stronger than butylene glycol; and comparable to propylene glycol, although each had its own advantages against given microorganisms.10-12
In addition, propanediol and butylene glycol were each tested for antimicrobial synergies with other preservatives in an o/w test formula. The formula was developed using 50% of the amount of preservative originally required to pass the antimicrobial challenge. In terms of Gram-positive and Gram-negative bacteria and mold antiseptic challenges, the synergistic antiseptic ability of propanediol was significantly higher than that of butylene glycol. When adding 6% propanediol to the test formula containing just 25% of its preservative system, the formula passed the antimicrobial challenge (not shown).13 Propanediol therefore acted synergistically with the existing preservative system, reducing the amount required.13
Additional Properties: Safety
Skin irritation and sensitization: The safety of propanediol was also evaluated through skin irritation and sensitization tests. Human repeat insult patch testing (HRIPT) was performed in 112 subjects using 5%, 25% and 50% aqueous solutions of propanediol. The test substance caused no irritation or sensitization to the skin at these concentrations (not shown).14
In another study, human repeat insult patch testing was performed in 207 subjects using 25%, 50% and 75% aqueous solutions of propanediol.14 At the 75% concentration, mild erythema was observed in only six of the subjects (not shown). From the experimental data, propanediol was deemed to cause almost no skin irritation or sensitization when the concentration is below 50%.
Similarly, an HRIPT safety comparison was made between propanediol and propylene glycol in 207 subjects using 5%, 25% and 50% aqueous solutions of propanediol and propylene glycol. It was found that 8.2% of subjects had a positive irritant reaction with 25% propylene glycol and this proportion of positive irritant reactions increased as the concentration increased. No positive irritant reaction was found with 75% propanediol (see Figure 11, below).14
Eye irritation: In an animal modelb, 0.1 mL of 100% propanediol was added to one eye in six animals without rinsing, then irritation was evaluated at 24 hr, 48 hr, 72 hr and seven days after treatment. Slight conjunctival redness was observed in four cases but it disappeared within 48 hr. The test substance was therefore not considered to be an eye irritant under the test conditions (not shown).15
In a separate animal modelb, 0.2 mL of 100% propanediol was added to one eye in each of four animals. Two treated eyes were rinsed while the remaining two were not, then eye irritation was evaluated after 30 min, 1 hr, 2 hr, 3 hr and seven days after treatment. Two animals whose eyes were flushed and one animal with an unflushed eye were observed to develop transient mild conjunctival redness that returned to normal after 48 hr. The propanediol was therefore not considered to be an irritant to the eyes under the test conditions (not shown).15 It was therefore concluded to cause no eye irritation, even if higher levels of it were used in a final makeup removal product.
HSP to predict eye irritation: It is worth noting that some scientific research institutions are currently predicting the eye irritancy potential of substances based on HSPs. The purpose is to seek alternatives to animal experiments, shorten substance screening time and explore the possibility of new methods to evaluate eye irritation. This concept uses the known Globally Harmonized System (GHS) category 1, 1(A) and 2(B) irritants to visualize a sphere (see Figure 12, below) based on HSPs, then compare with GHS category NC substances to verify eye irritation. Results have been up to 87.7% accurate,16 suggesting there are new methods to explore to determine the safety of substances in place of animal testing.
Additional Properties: Sensory Effects
Finally, a consumer panel (n = 20) tested and rated the sensory characteristics of an o/w lotion containing one of four glycols at 5%: butylene glycol, propanediol, propylene glycol or glycerin. The results are shown in Figure 13 (see below) and indicated propanediol outperformed the other poloyols for almost every parameter, but especially in terms of no tackiness, no film forming, a soft feeling, absorbing easily, feeling comfortable, feeling smooth and imparting an overall pleasant experience.17
Conclusion
With the continuous development of emulsification technologies, various new materials are being used in makeup removers; polyols especially have received much attention. The present article aimed to analyze, compare and logically derive – through existing technical theories and data – new methods and dimensions to aid formulators in choosing poloyls or other materials to advance formula designs.
As has been shown, various polyols have their own characteristics and advantages for application in makeup removers. This comprehensive comparison found that the hydrophilicity and lipophilicity of propanediol, in particular, is relatively intermediate among polyols. This characteristic, in addition to propanediol’s natural origin and additional skin and safety benefits, suggest its successful application in makeup remover waters, emulsions, bi-continuous makeup removers and other products.
Footnotes
a Zhuben is a brand of Hangzhou Shucai Network Technology Co., Ltd.
b Note that the author’s company, being based in China, is bound by regional regulations to perform given tests to ensure product safety; animal testing was required in this case.
References
1. Verified Market Research. (2024, Jul). Global makeup remover wipes market size by product type, by distribution channel, by material type, by geographic scope and forecast. Available at https://www.verifiedmarketresearch.com/product/makeup-remover-wipes-market/
2. Koda, Y., Sato, S. and Honma, Y. (1984). Chapter 1: The basic knowledge of organic concept. Organic Conceptual Diagram, Basis and Application. Sankyo: Tokyo.
3. Koda, Y., Sato, S. and Honma, Y. (1984). Chapter 2: The basic application of organic concept. Organic Conceptual Diagram, Basis and Application. Sankyo: Tokyo.
4. Hansen, C.M. (2007). Hansen Solubility Parameters. A User’s Handbook, second edition, CRC Press, Boca Raton; doi: https://doi.org/10.1201/9781420006834
5. LibreTexts Chemistry. (Accessed 2025, March 4). Chapter 17: Alcohols and phenols. UC Davis Library. Available at https://chem.libretexts.org/Courses/Athabasca_University/Chemistry_360%3A_Organic_Chemistry_II/Chapter_17%3A_Alcohols_and_Phenols
6. Sagitani, H., Ikeda, Y. and Ogo, Y. (1984, Mar 19) Effect of polyols on the association state of nonionic surfactants in aqueous solutions. SciSpace. Journal of Japan Oil Chemists’ Society. Available at https://scispace.com/papers/effect-of-polyols-on-the-association-state-of-nonionic-3eir03aygh
7. LibreTexts Biology. (Accessed 2025, Mar 4). Chapter 1.9 Functional Groups. Available at https://bio.libretexts.org/Courses/University_of_California_Davis/BIS_2A%3A_Introductory_Biology_(Britt)/01%3A_Readings/1.09%3A_Functional_Groups
8. Sagitani, H., Hirai, Y., Nabeta, K. and Nagai, M. (1986). Effect of types of polyols on surfactant phase emulsification. J-Stage. Journal of Japan Oil Chemists’ Society. Available at https://www.jstage.jst.go.jp/article/jos1956/35/2/35_2_102/_article
9. Fragrance Journal. (2009) 37(5) pp 61-64
10. Internal data from Zhangjiagang Glory Chemical Industry Co., Ltd.
11. Third-party testing by Japan Food Research Laboratory; commissioned by DuPont Tate & Lyle.
12. Guangdong Chem. (2017). 44(18) pp 89-91
13. Personal Care. (2012, Apr). pp 85-88
14. Belcher, L.A., Musha, C.F. and DeSalvo, J.W. (2010, May). Evaluating 1,3-propanediol for potential skin effects. Cosmetics & Toiletries. Available at https://cosmeticsandtoiletries.texterity.com/cosmeticsandtoiletries/library/page/201005/81/
15. DuPont Tate & Lyle Bio Products. (2007, Feb 22).
16. J. Soc. Cosmet. Chem. Jpn. 55(3) 288-297.
17. Durham, R.F., Miller, R., DeSalvo, J.W. (2010 Sept). Natural glycol replacement for hair and skin care. Personal Care. Available at https://rb.gy/ok4fbq