Propolis' natural variability challenges consistent extraction, so an alternative non-alcoholic technology was designed to optimize and standardize this. The resulting derm-grade propolis was tested for anti-inflammatory, anti-aging and other benefits, as described here.
Propolis is well-known as a resinous substance collected by honeybees, recognized for its rich array of bioactive compounds including flavonoids, phenolic acids and caffeic acid phenethyl ester (CAPE). These compounds possess powerful antioxidant, anti-inflammatory and antimicrobial properties, making it particularly valuable in dermatology care for wound healing, acne treatment and staving off infections. What’s more, its unique anti-inflammaging action can counteract the chronic low-grade inflammation linked to aging, supporting skin firmness, elasticity and an overall youthful appearance.
However, the natural variability in propolis’ composition has historically challenged its consistent extraction and production, impacting its reliability as a skin care ingredient and in formulations.16 Also, while traditional hydroalcoholic extraction methods have long been the standard for obtaining propolis, exposure to such solvents can degrade sensitive bioactives.
To address these issues, a patented non-alcoholic extraction technology (NEXT)a was developed, whose optimized mix of PEG 400 and soybean lecithins enables the chemoselective extraction and stabilization of critical bioactive compounds such as CAPE and phenolic acids. This approach, paired with suitable analytical methods, was used to create a stable and reproducible17 propolis extractb, positioning the ingredient as a high quality, potent, natural and reliable active for dermatological-grade skin care formulations. By preserving the integrity of the propolis bioactive compounds, the NEXT process also makes them more bioavailable, allowing for lower concentrations to deliver heightened efficacy for effects such as anti-inflammaging, wound healing, etc.
The present paper describes studies to characterize the active constituents in the derm-grade propolis extract, comparing results by traditional extraction versus the NEXT process. In addition, in vitro tests for wound healing, anti-inflammation and antimicrobial effects and clinical studies for anti-wrinkle benefits were carried out as described here.
Materials and Methods: Propolis Characterization, Anti-inflammation
Extraction process: The propolis extractb used in this study was obtained using the described NEXT process.
High-performance liquid chromatography (HPLC): The quantification of key active substances and auxiliary compounds was carried out using HPLCc. The mobile phase consisted of 0.1% formic acid in water (Phase A) and methanol (Phase B), with a gradient from 80% A/20% B to 20% A/80% B. UV-VIS was performed at 370 nm for detection and 290 nm for integration.
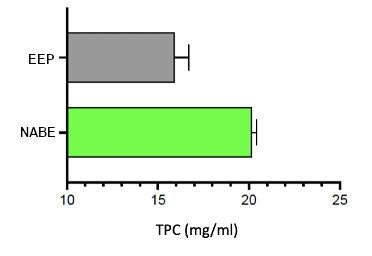
Total phenolic content (TPC): TPC was determined using the Folin-Ciocalteu assay on a 96-well microplate, measuring absorbance at 765 nm. The results were expressed in gallic acid equivalents, and compared the results of the traditional ethanolic extraction process (EEP) versus the NEXT process.
Anti-inflammation potential: The potential anti-inflammatory efficacy of the test propolis was assessed in a macrophage-mediated chronic inflammation (Mphg) system, wherein macrophages and venular endothelial cells were co-cultured and stimulated with a TLR2 ligand to model Th1-type chronic inflammation. Direct ELISA was used to measure biomarker levels of both cell-associated and membrane-bound targets. Soluble factors from supernatants were quantified using homogeneous time resolved fluorescence (HTRF) detection, bead-based multiplex immunoassay or capture ELISA.
Materials and Methods: Wound Healing Assay
To evaluate wound healing, the scratch assay was employed using HaCaT keratinocytes. The migration of cells to close the wound area was monitored over a 72-hr period, providing insights into the ability of different concentrations of the derm-grade propolisb to promote skin regeneration.
Clinical Test Protocol
A double-blind, randomized, controlled trial was conducted to assess the anti-wrinkle efficacy of the propolis extractb. Forty-seven female participants, ages 30-70 years, were assigned to either Product A (1.5% propolis) or Product B (3% propolis), which were leave-on facial emulsions (pH 4.5-5.5). They also contained: water (aqua), petrolatum, cetearyl alcohol, Paraffinum liquidum, ceteareth-20, benzyl alcohol, sodium phosphate, phosphoric acid and sodium hydroxide. The formulas were applied twice daily to the face and neck, and the wrinkle depth in the crow’s feet area was measuredd at days 0 and 28. Dermatological evaluations and participant questionnaires were used to assess the product’s efficacy, safety and consumer satisfaction.
Results: Propolis Characterization
As stated, achieving consistency in the concentrations of bioactives in propolis extract has been challenged by the material’s natural variability. The NEXT process addresses this by introducing a patented solvent ratio that stabilizes key bioactives, ensuring that each batch offers reliable and reproducible benefits regardless of source or quality of propolis as the starting material.16
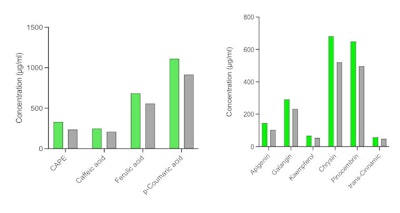
As shown by TPC, the patented extraction method yielded 20-40% more bioactive compounds than traditional EEP (see Figure 1 below), which would significantly improve the potency of the propolis extract in formulations. In addition, NEXT yielded greater key bioactive marker concentrations than EEP (see Figure 2 below).
Results: Anti-inflammation, Wound Healing Potential
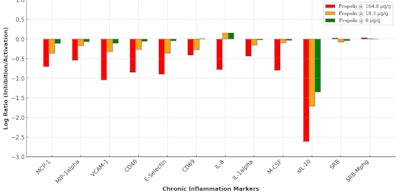
As shown in Figure 3 (below), propolis dose-dependently suppressed key inflammatory markers, including MCP-1, which attracts immune cells; MIP-1α, involved in immune cell recruitment; VCAM-1 for leukocyte adhesion; CD40 for immune activation; E-selectin, involved in inflammatory cell adhesion; the IL-1α pro-inflammatory cytokine; M-CSF for macrophage production; and sIL-10, involved in inflammation modulation.
At the highest concentration of 0.0165% w/w, a significant reduction in IL-8 levels was observed, alongside the most pronounced inhibition of sIL-10 and M-CSF, highlighting propolis’ anti-inflammatory action. IL-8, a key component of the senescence-associated secretory phenotype (SASP), plays a central role in chronic inflammation and the accumulation of senescent cells. The inhibition of IL-8 by propolis suggests that it may help modulate cellular senescence and reduce senescence-associated inflammation, potentially slowing the skin aging process.
Furthermore, cell proliferation assays such as SRB and SRB-Mphg, which measure cell proliferation by detecting protein content, also showed slight increases (see Figure 3 below). This suggests the propolis extract could modulate cell proliferation and support tissue regeneration during wound healing. Indeed, in vitro studies in dermal fibroblasts and keratinocytese described elsewhere20 showed the propolis increased collagen III and the proliferation marker SRB.
It is important to note that the observed discrepancy between the sample treatment and the vehicle control is primarily due to the differences in biomarker activity readouts, which are expressed as log-transformed ratios of test-agent-treated samples (n = 1) relative to vehicle controls (average of ≥ 6). Variability arises from biological and experimental factors, including the inherent properties of primary human cells, the specific responses to the stimuli in each system, and the effect of the standardized extract concentrations tested. This approach is standard for the analyses usede to quantify biomarker modulation relative to the control baseline.
Results: Wound Healing Assay
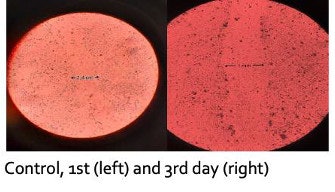
In HaCaT keratinocytes, the wound healing assay showed the propolis extract at 1.5% in PBS reduced the wound area by 70% within 48 hr. Complete wound closure was achieved at lower concentrations of 0.5% after 72 hr, confirming the ingredient’s skin-regenerative properties (see Figure 4 below).
Antimicrobial Efficacy
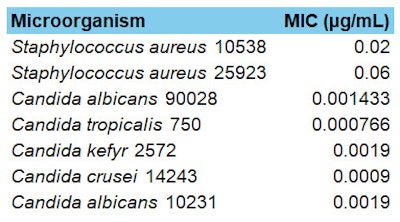
The antimicrobial activity of the propolis extract also was tested against key skin pathogens, such as Staphylococcus aureus and Candida albicans, both of which are implicated in skin infections and conditions such as acne, where bacterial overgrowth leads to inflammation. Table 1 (below) shows the Minimum Inhibitory Concentration (MIC), where lower MIC values indicate greater efficacy. As can be seen, the propolis extract exhibited strong antimicrobial efficacy, with particularly low MIC values against S. aureus and several Candida species.
Results: Clinical Testing
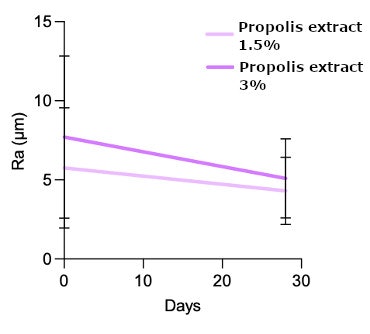
In clinical studies, the propolis extract demonstrated substantial anti-wrinkle efficacy. After 28 days of use, subjects saw a significant 25% reduction in wrinkle depth with 1.5% propolis, and 34% reduction with 3% propolis (see Figures 5 and 6, below; Wilcoxon rank-sum test, p < 0.01). Furthermore, 85% of participants expressed interest in purchasing the test creams, illustrating the market potential. Figure 6 shows the improvement in wrinkle depth reduction, which visibly smoothed and reduced fine lines.
Discussion and Conclusion
The development of an optimized propolis extractb using a novel non-alcoholic extraction technology (NEXT) process is described here. The process successfully addresses the challenge of variability in the composition of propolis extractsa, 17 to ensure consistent and reproducible concentrations of key bioactives like CAPE and phenolic acids.16, 17 The resulting propolis is presented as a dermatological grade ingredient for both therapeutic and cosmetic skin care applications, for which its efficacy was tested.
Clinical studies showed significant anti-aging effects, achieving wrinkle reductions of up to 34% after 28 days of application at 3%. Additionally, wound-healing efficacy was confirmed in vitro in a scratch test showing 70% closure within 48 hr and complete closure after 72 hr.
Anti-inflammatory properties also were particularly noteworthy. The extract significantly reduced IL-8, along with the pronounced inhibition of sIL-10 and M-CSF, indicating anti-inflammatory effects. Since IL-8 is a key component of SASP and plays a central role in chronic inflammation and the accumulation of senescent cells, by reducing IL-8 levels, the extract could also modulate cellular senescence, reduce senescence-associated inflammation and potentially slow the skin aging process.
The inhibition of sIL-10 and M-CSF also further supports the ingredient’s role in promoting skin repair and reducing the chronic low-grade inflammation that contributes to skin aging. In addition, its antimicrobial activity against S. aureus and C. albicans could be leveraged to manage skin infections and control pathogens for long-term skin health.
What’s more, 85% of clinical study participants expressed interest in purchasing the propolis test products, indicating the market opportunity. And in light of recent EU regulations21 surrounding retinol use, the ingredient’s anti-inflammatory and anti-wrinkle efficacy position it as an effective and gentle potential retinol alternative.
Taken together, the standardized, scientifically validated propolis extract is well-positioned as a cornerstone skin care ingredient for next-generation anti-aging, acne treatment and even post-procedure barrier repair – to not only combat visible signs of aging, but also regulate skin’s inflammatory response for overall skin health and well-being.
Footnotes
a WO2020169425A1, Apiotix Technologies
b Apinol360 (INCI: PEG-8 (and) Propolis Extract (and) Lecithin) is a product of ApiotiX Technologies.
c Ascentis Express C18 column (dimensions: 15 cm x 3.0 mm, particle size: 2.7 μm)
d PRIMOS-CR
e BioMAP
References
- Ahangari, Z., Naseri, M. and Vatandoost, F. (2018). Propolis: Chemical composition and its applications in endodontics. Iranian Endodontic Journal, 13(4), 285-292.
- Przybyłek, I. and Karpiński, T.M. (2019). Antibacterial properties of propolis. Molecules, 24(11), 2047.
- Bankova, V. (2005). Chemical diversity of propolis and the problem of standardization. Journal of Ethnopharmacology, 100(1-2), 114-117.
- Rufatto, L.C., Luchtenberg, P., ... Garcia, C., et al. (2018). Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiological Research, 214, 74–82.
- Do Nascimento, T.G., dos Santos Arruda, R.E., ... da Cruz Almeida, E.T., et al. (2019). Comprehensive multivariate correlations between climatic effect, metabolite-profile, antioxidant capacity and antibacterial activity of Brazilian red propolis metabolites during seasonal study. Scientific Reports, 9, 18293.
- Bankova, V., Popova, M. and Trusheva, B. (2006). Plant sources of propolis: An update from a chemist’s point of view. Natural Product Communications, 1(11), 1023-1028.
- Souza, E.A., Zaluski, R., Veiga, N. and Orsi, R.O. (2016). Effects of seasonal variations and collection methods on the mineral composition of propolis from Apis mellifera Linnaeus beehives. Brazilian Journal of Biology, 76(2), 396-401.
- Huang, S., Zhang, C.-P., Wang, K., Li, G.Q., and Hu, F.-L. (2014). Recent advances in the chemical composition of propolis. Molecules, 19(12), 19610-19632.
- Banskota, A.H., Tezuka, Y. and Kadota, S. (2001). Recent progress in pharmacological research of propolis. Phytotherapy Research, 15(7), 561-571.
- Daleprane, J.B. and Abdalla, D.S. (2013). Emerging roles of propolis: Antioxidant, cardioprotective and antiangiogenic actions. Evidence-Based Complementary and Alternative Medicine, 1-8.
- Sforcin, J.M. and Bankova, V. (2011). Propolis: Is there a potential for the development of new drugs? Journal of Ethnopharmacology, 133(2), 253-260.
- Fernandes-Silva, C.C., Salatino, A., Salatino, M.L.F., Breyer, E.D.H. and Negri, G. (2013). Chemical profiling of six samples of Brazilian propolis. Química Nova, 36(2), 237-240.
- Bankova, V.S., Christov, R.S. and Tejera, A.D. (1998). Lignans and other constituents of propolis from the Canary Islands. Phytochemistry, 49(5), 1411-1415.
- Batista, M.C.A., Abreu, B.V.B., ... Dutra, R.P., et al. (2016). Chemical composition and antioxidant activity of geopropolis produced by Melipona fasciculata in Maranhão State, northeastern Brazil. Acta Amazonica, 46(3), 315-322.
- Pellati, F., Prencipe, F.P., Bertelli, D. and Benvenuti, S. (2013). An efficient chemical analysis of phenolic acids and flavonoids in raw propolis by microwave-assisted extraction combined with high-performance liquid chromatography using the fused-core technology. Journal of Pharmaceutical and Biomedical Analysis, 81-82, 126-132.
- Šuran, J., Cepanec, I., Mašek, T., Radić, B. and Radić, S. (2021). Propolis extract and its bioactive compounds — From traditional to modern extraction technologies. Molecules, 26(10), 2930.
- Radić, S., Radić, B. and Šuran, J. (2020). Liquid propolis extract, its preparation and use thereof (WO Patent No. 2020169425A1). Google Scholar. Available at https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020169425.
- Šuran, J., Radić, B., ... Trevisan-Silva, D., et al. (2024). First proteome analysis of poplar-type propolis. Plant Foods for Human Nutrition, 79(1), 83-89.
- Mašek, T., Perin, N., ... Racané, L., et al. (2018). Chemical composition, antioxidant and antibacterial activity of different extracts of poplar-type propolis. Croatica Chemica Acta, 91(1), 81-88.
- Radić, B., Radić, S., Mašek, T. and Šuran, J. (2024). Anti-wrinkle efficacy of standardized phenolic acids polymer extract (PAPE) from propolis: Implications for anti-aging and skin health. Journal of Cosmetic Dermatology.
- Cosmetics & Toiletries. (2024, Jul 11). 9 Cosmetic ingredient bans/restrictions in the EU: Kojic acid, 4-MBC, retinol, arbutin and more. Available at https://www.cosmeticsandtoiletries.com/regulations/regional/news/22913539/ctpa-9-cosmetic-ingredient-bansrestrictions-in-the-eu-kojic-acid-4mbc-retinol-arbutin-and-more