Editor’s note: This article is the tenth in a series covering the EU requirements for cosmetic products; see the previous: Part I (European Regulations), Part II (Responsible Person), Part III (PIF compliance), Part IV (Cosmetic Product Safety Report), Part V (data mandate), Part VI (risk assessment), Part VII (sensitization test), Part VIII (preservatives) and Part IX (stability).
European Commission (EC) Cosmetics Regulation No. 1223/20091 is the main regulatory framework for finished products placed on the EU market (see Part I). It states that cosmetic products must be safe for human health, and requires products to undergo a safety assessment. The safety of cosmetic products is based on the safety of their ingredients.2, 3 Thus, their toxicological profile must be evaluated (see Part VI).
Genotoxicity is one of the critical endpoints used to assess cosmetic ingredient safety, as highlighted in the regulation guidelines.2 Further, since 2013, the regulation requires testing to be performed using non-animal methods. This is the focus of the present article in our series.
Genotoxicity Assessment
Genotoxicity refers to processes that alter the structure, information content or segregation of DNA. Compared to most other types of toxicity, genetic alterations may result in effects manifested after unusually long periods following exposure. For an adequate evaluation of the genotoxicity potential, three endpoints must be assessed:3
- Gene mutation (mutagenicity)
- Structural chromosomal aberrations (clastogenicity)
- Numerical chromosomal aberrations (aneugenicity)
In vitro Tests for Genotoxicity
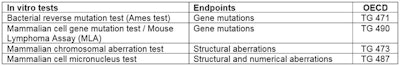
The Organization for Economic Co-operation and Development (OECD) has validated four in vitro tests to assess the three endpoints and provided test guidelines (TG) (see Table 1). The Ames test is the most commonly used.
Test Battery Strategy
To adequately cover all the genetic endpoints, one must use several tests (i.e., a test battery) since no single test can provide information on all endpoints. The Scientific Committee on Consumer Safety (SCCS) of the EC recommends3 two in vitro tests for the base level testing of cosmetic substances: the Ames test and micronucleus test.
If both tests are negative, it is very likely the substance has no mutagenic potential. If both tests are positive, it is very likely that the substance has mutagenic potential. In both cases, no further testing is necessary. If one test is positive, the substance is considered an in vitro mutagen and further testing can be used to better assess the mutagenic potential.
Four in vitro tests are validated to assess the genotoxicity potential of cosmetic ingredients.
The four scenarios possible are presented in the Addendum to the SCCS's Notes of Guidance, 8th Revision,4 and if needed, further strategies are proposed. In any case, a weight of evidence (WoE) approach must be considered if the previous Ames + micronucleus strategy did not provide a clear answer.
It is important for cosmetic manufacturers to gather the safety data, including genotoxicity, of each ingredient in their formulations since it will be critical in the assessment of their product by the safety assessor.
References
All websites accessed June 23, 2017.