A previous "Comparatively Speaking" column, Homologous vs. Analogous Polymers,1 discussed the relationship between related analogous and homologous series of chemicals for predicting their functional and formulation properties.1 This article made it clear that the solubility of an alkyl dimethicone is directly related to the amount of alkyl group (by weight) in the polymer. This information is critical to the efficient selection of a particular alkyl dimethicone used in a formulation.
Taking this observation a step further, the present column proposes a modification to the time-honored HLB system to extend to the function of alkyl dimethicone compounds in silicone fluid, mineral oil and esters.
Alkyl Silicone Structure and Solubility
Specifically, the prior column had addressed a series of alkyl silicones with different w/w percentages of alkyl group present. The structure is shown in Figure 1,
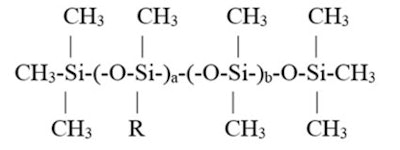 where R is –(CH2)15–CH3. These percentages were altered by changing the a and b values, which resulted in a series of products that were found to be soluble in either: 350-viscosity silicone fluid, mineral oil or C8-10 triglyceride. A graphic representation is shown in Figure 2.
where R is –(CH2)15–CH3. These percentages were altered by changing the a and b values, which resulted in a series of products that were found to be soluble in either: 350-viscosity silicone fluid, mineral oil or C8-10 triglyceride. A graphic representation is shown in Figure 2.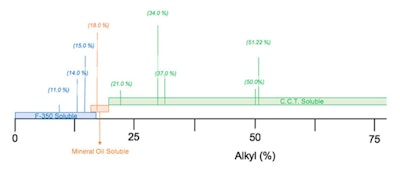 Upon closer examination, the graphic in Figure 2 looks similar to a chart depicting the hydrophilic-lipophilic balance (HLB)2, 3 if the hydrophilic group were replaced with silicone. In other words, a siliphilic-lipophilic balance (SLB) system.
Upon closer examination, the graphic in Figure 2 looks similar to a chart depicting the hydrophilic-lipophilic balance (HLB)2, 3 if the hydrophilic group were replaced with silicone. In other words, a siliphilic-lipophilic balance (SLB) system.SLB System
If we define the SLB as: SLB = % alkyl/5, anything below 4 would be predicted to be silicone-soluble; anything below 4 and 5, mineral oil-soluble; and anything above 5 should be soluble in C8-10 triglyceride. This classification allows formulators to select an alkyl dimethicone that is soluble in silicone fluid, mineral oil or esters.
Further, materials that are close to being soluble in both will help maintain a dispersion that contains all three. The proper understanding of which alkyl silicone works in which formulation requires an understand of the structure/function properties of the alkyl silicone chosen and the ingredients in the oil phase. The use of the SLB system therefore provides a metric for this selection.
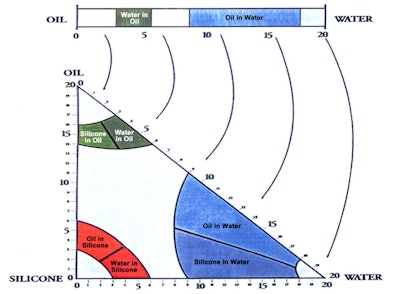
Three-dimensional HLB
In relation, the three-dimensional (3D) HLB system first published in 1996 addressed non-traditional emulsions and the silicone polymers that would facilitate making them (see Figure 3).4 The two proposed emulsions were oil-in-silicone (o/s) and silicone-in-oil; and in fact, the predicted % alkyl for o/s emulsions was between 3 and 6.
 Conclusion
ConclusionThe ability to efficiently formulate products using alkyl dimethicone polymers in personal care requires knowing: the composition of those polymers, their solubility in the oil phase chosen, and a metric to understand which values are necessary for that particular oil phase. SLB is a starting point for such determinations.