The skin is a complex and dynamic organ able to perform several physiological functions. The primary function is to provide a physical barrier that maintains an adequate level of hydration while protecting the body from exogenous substances such as bacteria, allergens and pollution. Moreover, the skin provides protection from UV rays, regulates body temperature and is involved in all sensory perception.
However, skin maturation is a gradual process for the first two years of life in which level of maturity is a function of gestational age. This process begins at birth, when the skin suddenly must adapt to a relatively dry environment, compared with in utero surroundings. Furthermore, in newborns, neither sebaceous nor sweat glands function maturely.1–4
The present column considers infant skin biology during a time when compromised skin conditions are on the rise. It also proposes formulating solutions to meet the specific needs of this immature skin state.
Infant Skin Biology
Since skin maturation is a continuous process, when compared with fully developed skin, infant skin differs in various ways.
- It is thinner by 20–30%; the epidermis, in particular, is under-keratinized, compared with mature skin.
- It has higher water content, but, at the same time, lower water retention capacity—which means higher transepidermal water loss (TEWL), particularly in the early months of life. Within approximately three months, stratum corneum hydration increases and can even exceed hydration levels in mature skin.5–8
- Its hydrolipidic film is immature—due to lower amounts of sebum and total lipids than in mature skin, as well as the low expression of natural moisturizing factors—leading to the aforementioned low water-retention capacity.
- Its pH level is higher than that of mature skin. At birth, newborn skin is more alkaline, ranging from pH 6–7.5 depending on the anatomical site.7, 8 This acidity not only limits the growth of pathogenic skin flora, but also is required for the enzymatic lipid processing that results in the development of an effective permeability barrier. Low pH values inhibit the colonization of pathogens such as Staphylococcus aureus and Streptococcus pyogenes, while encouraging the growth of resident skin flora.9–11
- Its melanization is low, which is of considerable importance since melanin functions as a UV filter, reducing the penetration of UV light through the epidermis. Lower melanin concentration—together with thinner stratum corneum, increased stratum corneum hydration and presumably, reduced light-scattering—could potentially contribute to a heightened sensitivity to the harmful effects of UV radiation.12, 13
All of these elements highlight key challenges to this specialized, evolving state of the skin: reduced barrier function, increased risks of irritation and penetration, and increased susceptibility to microbial contamination.
According to this picture, it is crucial to deliver appropriate care to infant skin, rightly tailoring solutions to the needs of the growing epidermis.
Compromised Conditions on the Rise
In relation, in industrialized countries, the percentage of individuals afflicted with various skin conditions has tripled in the past 30 years. Atopic dermatitis (AD), for example, is the most common skin disease observed in industrialized countries; and it strikes all populations.
One recent study indicated incidences of 17% in black and 15% in Hispanic individuals. Another study showed that from 2000 to 2010, the index for AD in individuals younger than 18 rose from 9–17% in black, 5–10% in Hispanic, and 8–13% in white children.14 These increases are most certainly linked to a worsening environment, lifestyle changes and excessive hygiene habits.
As described, infant skin is exposed to greater risks than mature skin—in addition to the body having an under-developed immune system. For this reason, the chances of being affected by AD in the first quarter of life are quite high.
Symptoms and Sites
The most common symptom of AD is a dry and itchy skin rash. For this reason, AD has been called the “itch that rashes.”15 Such pruritus can lead to scratching until the skin bleeds, which in turn can aggravate the disease and cause a vicious cycle of redness, swelling, cracking, weeping clear fluid, crusting, thick skin and scaling.16
AD is often found on the face, inside the elbows, behind the knees and on the hands and feet. Around the age of one, AD is most common in the folds of the skin—i.e., neck, elbows, behind the knees and under the arms. At the ages of three and four it also mainly affects skin folds, but additionally the hands and face, around the mouth and on the eyelids. At the ages of five and six, strong exacerbations disappear but the skin dryness tends to persist.
Skin affected by AD could therefore benefit from simple preventative measures such as proper clothing, as well as tailored pharmacological therapies and, of course, appropriate hygiene and moisturizing treatments.
Infant skin poses challenges ranging from reduced barrier function, higher water content yet lower retention capacity, and higher pH, among others.
Cleansing Formulations
Cleansing and hygiene obviously occur frequently during the first months of life. From seven to eight (or more) diaper changes per day, to bathing and shampooing, proper cleansing practices are essential to avoid skin problems and, in the case of AD management, to remove debris, crust, exudates and microbial agents, which can cause skin infection and trigger irritation. During cleansing routines, hot water should be avoided to prevent both vasodilation—which can trigger pruritus—and damage. Additionally, hard water, which is slightly alkaline and high in salt content, can be an irritant due to its pH and impact on the skin barrier.
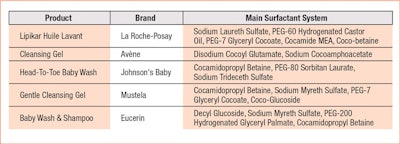
Wet wipes and cleansing bath and shower gels must be formulated so as to avoid any further potential damage to infant skin. The selection of the right surfactant, e.g., anionic, ethoxylated, amphoteric, etc., is important, but the correct combination, i.e., ratio, percent active matter, and primary and secondary surfactants appears to have the greatest influence. Studies and reviews have described the interactions between surfactants and the skin17–20 and have attempted to link the surfactant physicochemical properties to skin toxicity.
In some cases, toxicity has been connected with the ability of a surfactant to form micelles, since monomers appear to cause skin irritation; as consequence, surfactants with high critical micelle concentration values were reported to be more toxic.21 In other studies, micelles appeared to be capable of penetrating the stratum corneum. Therefore, the size and shape of micelles should be considered.22, 23 If the mildness of different surfactant systems is measured using transepithelial permeability (TEP), the results show how mixed micelles—i.e., those composed by different surfactants—in solution can have different properties (see Figure 1).
Here, the surfactant system of the adult shampoo disrupts tight junctions between skin cells at the lowest product concentrations, indicating aggressive properties. The surfactants in the body wash are milder, inducing disruption but at somewhat higher concentrations. In contrast, the surfactant combination of the baby cleanser is well-tolerated by skin’s tight junctions over a broader range of concentrations.24 Indeed, the right surfactant combination provides the best option because, as the results of a HET-CAM test reveal (see Figure 2), even the mildness of a non-ionic surfactant alone can be questionable.25 The addition of the mild surfactant olivoyl fructoside (OF) is shown here to reduce the irritation potential of the traditional surfactant sodium laureth sulfate (SLES).26 This is why many of today’s baby cleansers are provided in various combinations (see Table 1). In some cases, especially for atopic-prone skin, the use of a system rich in refatting agents or emollients is preferred (see Formula 1 and Formula 2).
Moisturizing Treatments
Clinical evidence has shown that the management of many skin diseases can be optimized through complementary pharmacological and cosmetic treatments. Together, they relieve symptoms and prevent acute exacerbations while improving skin’s appearance, ultimately enhancing the quality of life in patients.27–29 Multiple guidelines recommend moisturizing therapies as the first step in treating AD.30–32 However, the distinction between moisturizers and topical barrier repair products is not clearly defined.
Often, moisturizer products available over-the-counter (OTC) are purely occlusive, e.g., petrolatum and lanolin, or contain both occlusive agents and humectant ingredients, e.g., glycerin. The main target of these products is to reduce TEWL and, consequently, increase stratum corneum hydration. Two inconveniences of this mechanism can be the excessive reduction of skin-breathing capability, or the quick return to a water loss condition once the occlusive mixture is removed.
Indeed, according to the literature, an excessive increase in stratum corneum hydration afforded by the application of occlusive substances does not lead to the well-being and protection of the skin. On the contrary, intense dermatitis can occur simply due to prolonged water exposure.33, 34 When occlusive substances are removed or reduced, the water from accumulated perspiration then evaporates so quickly that leads to cracked skin, rendering it susceptible to penetration by exogenous substances, allergens and irritants.35, 36 In conclusion, truly functional barrier recovery is correctly achieved if the product applied to skin is permeable to water; in contrast to occlusion, vapor-permeable membranes allow the barrier function to recover normally.
As such, the protective barrier provided by baby care products should resemble the vapor-permeable and protective barrier offered by the vernix caseosa—i.e., the protective emulsion covering the fetus during pregnancy. The aim of this layer is to guarantee the delicate balance of hydration in the unborn infant’s under-developed skin while immersed in amniotic liquid.
In the past decade, numerous studies have provided sufficient evidence of the beneficial properties of vernix caseosa. It acts as a barrier system to not only perfectly moisturize the skin, but also improve skin barrier recovery. It allows enzymes to function properly37 and appears to form a semi-occlusive barrier that overlays the developing stratum corneum.38
Greater risk factors and a weaker immune system make
the chances of being affected by AD in
the first quarter of life quite high.
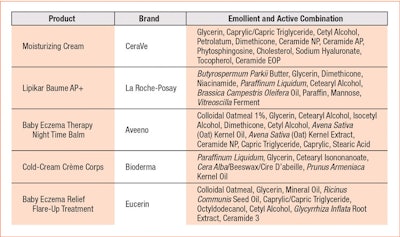
To meet similar needs in impaired infant skin, effective barrier repair formulas should contain the ingredients of both a conventional moisturizer—i.e., a mixture of occlusive petrolatum or mineral oil with non-occlusive oils, such as esters and triglycerides—plus, specific physiological ingredients such as ceramides and essential fatty acids in an emulsion structured to directly target skin barrier; e.g., lamellar or liquid crystal.39, 40 Contrary to the variety of surfactants used in commercial baby cleansing products, marketed moisturizing creams use more or less the same approach (see Table 2).
It is evident from this sampling that a common lipidic phase consists of a combination of petrolatum and Paraffinum liquidum with natural or synthetic triglycerides. Humectants, sodium hyaluronate, vitamins and antioxidants serve as actives. Some brands are also beginning to introduce ingredients that act on the microbiome.41
Conclusions
In the first years of life, the physiological characteristics of immature infant skin require a conscious selection and combination of ingredients in order to obtain mild cleansing with adequate protection and treatment. The purity of raw materials, e.g., levels of impurities such as heavy metals, peroxides, etc., must also be carefully checked during product manufacturing.
In fact, too natural a product may not be the right choice insofar as skin tolerability to variations in the raw materials, high levels of impurities, the instability of vegetable oils, etc. A mixture between natural, nature-like and synthetic could help to achieve an appropriate skin treatment.
Lastly, conventional preservatives such as potassium sorbate and parabens should be avoided due to their irritation/sensitization potential—bearing in mind that non-conventional preservatives including glycols and diols must be accurately considered due to irritation potential and penetration-enhancing effects.
References
- Nikolovski et al, Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life, J Invest Dermatol 128(7) 1728–1736 (2008)
- L Brancaleon et al, Attenuated total reflection-Fourier transform infrared spectroscopy as a possible method to investigate biophysical parameters of stratum corneum in vivo, J Invest Dermatol 116(3) 380–386 (2001)
- P Agache, D Blanc, C Barrand and R Laurent, Sebum levels during the first year of life, Br J Dermatol 103(6) 643–9 (1980)
- H Behrendt and M Green, Drug-induced localized sweating in full-size and low-birthweight neonates, Am J Dis Child 117(3) 299–306 (1969)
- J Nikolovski, GN Stamatas, N Kollias and BC Wiegand, Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life, J Invest Derm 128(7) 1728–36 (2008)
- S Saijo and H Tagami, Dry skin of newborn infants: Functional analysis of the stratum corneum, Pediatr Dermatol 8(2) 155–9 (1991)
- PH Hoeger and CC Enzmann, Skin physiology of the neonate and young infant: A prospective study of functional skin parameters during early infancy, Pediatr Dermatol 19(3) 256–62 (2002)
- G Yosipovitch, A Maayan-Metzger, P Merlob and L Sirota, Skin barrier properties in different body areas in neonates, Pediatrics 106(1 pt 1) 105–8 (2000)
- SM Puhvel, RM Reisner and M Sakamoto, Analysis of lipid composition of isolated human sebaceous gland homogenates after incubation with cutaneous bacteria. Thin-layer chromatography, J Invest Dermatol 64 406–411 (1975)
- JP Hachem, D Crumrine, J Fluhr, BE Brown, KR Feingold and PM Elias, pH directly regulates epidermal permeability barrier homeostasis and stratum corneum integrity/cohesion, J Invest Dermatol 121 345–353 (2003)
- HC Korting, A Lukacs, N Vogt, J Urban, W Ehret and G Ruckdeschel, Influence of the pH value on the growth of Staphylococcus epidermidis, Staphylococcus aureus and Propionibacterium acnes in continuous culture, Zentralbl Hyg Umweltmed 193 78–90 (1992)
- MC Mack, NK Tierney, E Ruvolo, Jr, GN Stamatas, KM Martin and N Kollias, Development of solar UVR-related pigmentation begins as early as the first summer of life, J Invest Dermatol (2010)
- KH Kaidbey, PP Agin, RM Sayre and AM Kligman, Photoprotection by melanin—A comparison of black and Caucasian skin, J Am Acad Dermatol 1(3) 249–60 (1979)
- fondation-dermatite-atopique.org/en (accessed Jul 30, 2018)
- SP Romeo, Atopic dermatitis: The itch that rashes, Pediatr Nurs 21 157–163 (1995)
- AC Krakowski and L Bennett, Topical therapies for atopic dermatitis. An update on topical management options and adjuvant interventions for this common condition, Practical Derm (Mar 2015)
- A Mehling, M Kleber and H Hensen, Compartive studies on the ocular and dermal irritation potential of surfactants, Food and Chem Tox 45 747–758 (2007)
- LD Rhein, CR Robbins, K Frernee and RJ Contore, Surfactant structure effects on swelling of isolated human stratum corneum, J Soc Cosmet Chem 37 125 (1986)
- MM Rieger, Surfactant interactions with skin, Cosm & Toil 110(4) 31–50 (1995)
- LD Rhein, Review of properties of surfactants that determine their interaction with stratum corneum, J Soc Cosmet Chem 48 253–274 (1997)
- M Corazza, M Lauriola, M Zappaterra, A Bianchi and A Virgili, Surfactants, skin cleansing protagonists, J Eur Acad Dermatol Venereol 24 1–6 (2010)
- PN Moore, S Puvvada and D Blankschtein, Challenging the surfactant monomer skin penetration model: Penetration of sodium dodecyl sulfate micelles into the epidermis, J Cosmetic Sci 54(1) 29–46 (2003)
- PN Moore, A Shiloach, S Puvvada and D Blankschtein, Penetration of mixed micelles into the epidermis: Effect of mixing sodium dodecyl sulfate with dodecyl hexa(ethylene oxide), J Cosmetic Sci 54(2) 143–159 (2003)
- RM Walters, M Fevola, J LiBrizzi and K Martin, Designing cleansers for the unique needs of baby skin, Cosm & Toil 123(12) 53–60 (2008) available at https://bit.ly/2v2zAEe
- sciencedirect.com/science/article/pii/S0927775715000382?via%3Dihub (accessed Jul 30, 2018)
- L Rigano, N Lionetti, A Bonigli, G Rastrelli and A Baratto, Testing and developing a sugar-derived surfactant blend for delicate skin, Cosm & Toil 129(6) 30-41 (Jul/Aug 2014) available at https://bit.ly/2ApuhDL
- EL Simpson, Atopic dermatitis: A review of topical treatment options, Curr Med Res Opin 26(3) 633-40 (Mar 2010)
- M Loden, Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders, Am J Clin Dermatol 4(11) 771–778 (2003)
- KL Hon, GK Ching, TF Leung, CY Choi, KK Lee and PC Ng, Estimating emollient usage in patients with eczema, Clin Exp Dermatol 35 22-6 (2010)
- CA Akdis, M Akdis, T Bieber T et al, Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report, J Allergy and Clin Immunol 118(1) 152–169 (2006)
- JM Hanifin KD Cooper, VC Ho et al, Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association, “Administrative Regulations for Evidence-Based Clinical Practice Guidelines,” J Amer Acad Derm 50(3) 391–404 (2004)
- nice.org.uk/nicemedia/pdf/cg057niceguideline.pdf (accessed Jul 30, 2018)
- F Terranova, Fisiopatologia dell’ idratazione cutanea, Medicina Dermatologica (2006)
- I Willis, The effects of prolonged water exposure on human skin, J Invest Dermatol 60 166–171 (1973)
- RR Warner, YL Boissy, NA Lilly, MJ Spears, K McKillop, JL Marshall and KJ Stone, Water disrupts stratum corneum lipid lamellae: Damage is similar to surfactants, J Invest Dermatol 113(6) 960–6 (Dec 1999)
- E Proksch, J Brasch and W Sterry, Integrity of the permeability barrier regulates epidermal Langerhans cell density, Br J Dermatol 134(4) 630–8 (Apr 1996)
- JW Wiechers and B Gabard, Vernix caseosa: The ultimate natural cosmetic? Cosm & Toil 124(9) 36–55 (2009) available at https://bit.ly/2LOPxY9
- W Youssef, SB Hoath and RR Wickett, In vitro water transport through vernix caseosa compared to Aquaphor and petrolatum, AAPS Journal (S1)2206 (2000)
- JQ Del Rosso, Moisturizer and barrier repair formulations, in ZD Draelos, ed, Cosmeceuticals, 3rd edn, Elsevier, Philadelphia (2016) pp 81–89
- ZD Draelos, The effect of ceramide-containing skin care products on eczema resolution duration, Cutis 81(1) 87–91 (Jan 2008)
- allure.com/gallery/probiotics-skin-care-products (accessed Jul 30, 2018)