Intercellular lipids of the stratum corneum (SC) form a lamellar structure (LS) that consists mainly of ceramides, cholesterols and fatty acids as amphiphilic substances, and that demonstrates significant skin barrier function.1, 2 This natural liquid crystalline (LC) structure has a bi-continuous composition of water and amphiphilic lipids, and simultaneously possesses high moisturizing and water loss prevention effects.3
In the past, much work has been devoted to preparing emulsions using the self-organization of structures formed in mixed systems of surfactants and amphiphilic lipids—such as fatty alcohols, fatty acids, lecithin, polyglycerol alkyl ethers and mono alkyl phosphates, among others—to develop bio-mimetic LC emulsions.4-7 In relation, Suzuki et al. disclosed work in this field describing a bio-mimetic lamellar LC system based instead on synthetic ceramides.8 Figure 1 exemplifies a schematic illustration of oil droplets surrounded by liquid crystals, forming LC o/w emulsions.
It has been reported that the advantages to using this type of system include higher moisturizing effects, as well as enhanced barrier function of the SC.2, 4, 5 However, few successful systems exist due to the poor stability of the LC structure during application to the human skin; i.e., the liquid crystals surrounding the oil droplets break and disappear quickly at skin temperature. Thus, the aim of the present study was to produce an optimized system for the preparation of LC o/w emulsions that are stable at temperatures higher than that of the skin, and to confirm their efficacy and function as bio-mimetics of intercellular skin lipids.
Materials and Methods
Materials: To prepare the LC base (LCB), the following materials were obtaineda: phytosterol (PS), PEG10-30 soy sterol (PEG10-30-SS), hydrogenated lecithin (HL), oils including squalane and caprylic/capric triglyceride, and waxes such as fatty alcohol. (See Figure 2). All materials were technical grade and used without further purification.
Preparing the LCB: The LCB was prepared in a wax form by mixing the HL, PEG10-30-SS, PS and fatty alcohols (C16-C22) at 80°C, a temperature above their melting points, then cooling the batch to room temperature. After several trial variations, the optimized weight ratio of each component was determined to be 1:2:1:6.
Phase diagram study: Various amounts of the LCB, oil (squalane) and water were sealed in ampoules. The phase states and presence of liquid crystals at 25°C were determined by direct visual inspection and polarization microscopy, respectively.
Repeat distance of the LC system: The interlayer spaces of the lamellar structure were examined using small angle x-ray scattering (SAXS)b with a Cu-Kα wavelength of λ = 0.1542 nm at 25°C. Two test samples were measured—an LCB aqueous system, 20% w/w, and the same concentration LCB in water with 20.0% w/w squalane system. Their LC phases were distinguished by SAXS peaks. Specifically, the ratios of interlayer spaces (d; repeat distance) from the first to the second and third peaks were 1:1/2:1/3 for the lamellar type.
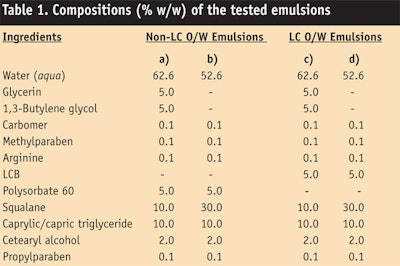
Preparing LC o/w and control emulsions: LC o/w emulsions were prepared with the LCB, oils, polyols and water by high speed homogenizing emulsification at 70 ± 5°C using a homomixerc. The LCB was first added to the oil phase and agitated to dissolve. This mixture of LCB with oils was then added into a water phase to obtain LC o/w emulsions. Meanwhile, non-LC o/w emulsions were prepared as controls, using polysorbate-60 as an emulsifier and following the same mixing procedure.
Confirming the structure of LC o/w emulsions: The structure of LC o/w emulsions was confirmed by two types of observation: polarized light microscopy attached to a digital camerad and transmittance electron microscopic (TEM) analysise. The structural stability also was confirmed at 30°C, 35°C, 37°C and 40°C.
Verifying liquid crystal stability on skin: The structural stability of LC o/w emulsions on the skin was confirmed by applying 20 μg/cm2 of LC o/w emulsions on the inside forearms of volunteers as described below; samples were left on the skin for 6 hr, after which the emulsions were recovered by tape stripping. The recovered test emulsion on the tape was transferred to a slide glass and observed by polarized light microscopy to determine whether the LC structures remained present.
Measurement of water content: Bound water content within LC o/w emulsions or non-LC o/w emulsions was measured by differential scanning calorimetry (DSC) analysisf.9
Clinical evaluation of moisturizing effect: 2.5 mg/cm2 of LC o/w emulsions or non-LC o/w emulsions were applied on the inside forearms of 6 volunteers, 25-45 years. The volunteers were first initialized for 15 min in an incubation room at 22°C and 45% relative humidity (RH). The conductance (µS) of the applied and non-applied areas was measured at 22°C and 45% RHg. Measurements were taken initially and at 15 min, 30 min, 45 min and 60 min after application. The moisturizing effect was evaluated by comparing the changes of conductance between the initial measurement and each time interval.10, 11
TEWL-reducing effect: LC o/w emulsions and non-LC o/w emulsions were applied twice daily on the inner thighs of 12 male volunteers, 28–55 years, for 21 days and the TEWL of the area was then calculated. Measurements were taken initially and at 1 week, 2 weeks and 3 weeks after application. The TEWL was evaluated using a water loss meterh in the incubation room maintained at 21°C and at 50% RH.12 The skin condition of the inner thighs before and after application was evaluated by tape-stripping a few layers of the SC using cellophane tape. The skin condition was observed by BG dye method.13
Results and Discussion
Phase diagram study: The schematic phase diagram of the LCB/squalane/water system in the whole concentration range at 25°C is shown in Figure 3. Confirmation of phase states depending on the compositions was determined by a polarization microscope. As shown in the water/LCB line, a lamellar liquid crystal phase is formed at 3–30% LCB, and this lamellar phase was confirmed to change to a hexagonal phase with increasing concentrations of LCB.
This hexagonal phase was present at up to 70% LCB but above 70%, a reverse micellar phase having a gel formation appeared. The lamellar liquid crystal phase that was formed in the range of 11–30% LCB was too viscousj and, according to evaluation by 5 panelists, felt too greasy for cosmetic formulations. Therefore, the most suitable condition for lamellar phase formation was determined to be in the range of 4% to 10% of LCB.
Repeat distance of the LC system: The SAXS measurement of the 20% LCB aqueous system showed three sharp peaks with interlayer spaces (d) of:
5.712 nm, 2.870 nm and 1.917 nm, whose ratios from the first to the second and third peaks were 1:1/2:1/3. The same concentration of LCB in water with 20.0% squalane system showed similar interlayer spaces (d) of:
5.770 nm, 2.885 nm and 1.923 nm, having the ratios of 1:1/2:1/3. Therefore, the liquid crystal system prepared with the LCB was characterized as a lamellar structure.
Confirming the structure of LC o/w emulsions: Test samples of LC o/w emulsions or non-LC o/w emulsions (see Table 1) were observed first by polarized light microscopy to confirm the existence of liquid crystalline structures. As seen in Figure 4, LC o/w emulsions showed the Maltese cross image of emulsion droplets (Figure 4c-d); however, non-LC o/w emulsions did not (Figure 4a-b). Therefore, LC o/w emulsions based on the LCB were confirmed as having liquid crystalline structures that could be identified as a lamellar form. On the other hand, non-LC o/w emulsions prepared with polysorbate-60 did not form a liquid crystalline structure.
The structure of liquid crystals surrounding the oil droplet of LC o/w emulsions (Table 1c) was then observed by TEM. As shown in Figure 5, the existence of multi-lamellar structures around oil droplets was confirmed (Figure 5a) and this multi-lamellar structure is similar to intercellular lipid layers in the SC (5b).14, 15 This result can be explained by the equation below, known as the critical packing parameter (CPP):
CPP = v/(ao lc) Eq. 1
where ao is the optimal surface area; v is the hydrocarbon chain volume; and lc is the critical length of the lipids.
The CPP determines whether lipids will form spherical micelles (CPP<1/3), nonspherical micelles (1/3
Finally, the similarities between the LCB system and SC lipids were compared. Previous studies indicate that SC lipids have an LC structure that consists mainly of ceramides, cholesterols and fatty acids.1, 2 Moreover, the schematic diagram of the proposed model of lamellar phase formation of the epidermal barrier has been reported as well.16 Therefore, although the individual components of the LCB do not have exactly the same structure as SC lipids, the LC system with the LCB having a lamellar form can be regarded as showing great similarities to SC lipids.
Stability of the LC Structure
Temperature stability: The stability of LC o/w emulsions (see Table 1c) at different temperatures is shown in Figure 6. Each image shows a change in the LC form as the temperature increases from 30°C to 35°C, 37°C and 40°C, confirming that the LC form is maintained even up to 40°C-higher than the normal skin temperature of ~33°C. Therefore, the LC o/w emulsions could be expected to maintain their LC form in practical applications on the skin surface.
Presence of LC on skin: As shown in Figure 7, the recovered LC o/w emulsions (see Table 1c; applied amount = 20 µg/cm2) from the inside forearms of volunteers demonstrated Maltese cross images after 6 hr, suggesting the LC form is stable against skin temperature. Therefore, LC o/w emulsions were concluded to act as an artificial SC lipids.
Bound water content: Generally, water within intercellular lipids, or bound water, exists in a different form than free water, which easily evaporates from the skin surface. Bound water in intercellular lipids is retained tightly in the lipid structure and protects the skin against over-drying.
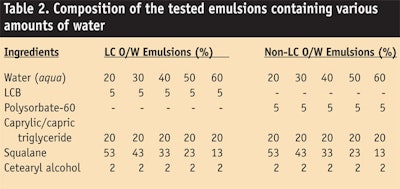
The bound water content in LC o/w emulsions (see Table 2) was quantified and compared with the water content in non-LC o/w emulsions by enthalpy measurements of melted test samples containing various amount of water—20%, 30%, 40%, 50% and 60%. Here, total content of water (bound water + free water) in the test samples was quantified by the Karl Fisher method. The results are shown in Figure 8.
Figure 8a depicts the relationship between the enthalpy changes and the total water content. The y axis gives an estimate of the bound water content; the LC o/w emulsions showed that the
y axis was 12.7%, and the correlation coefficient (R2) was 0.9968. In non-LC o/w emulsions, the y axis was 8.3%, and the correlation coefficient (R2) was 0.9967. According to the results, LC o/w emulsions were estimated to have almost 53% more bound water than non-LC o/w emulsions. Consequently, LC o/w emulsions were expected to provide better moisturization than non-LC o/w emulsions.
Clinical Evaluation
Moisturizing effect: The moisturizing activity of LC o/w emulsions (see Table 1c) was evaluated and compared with non-LC o/w emulsions (see Table 1a) by measuring the skin conductance of the volunteers. Figure 9 shows the changes in skin conductance as a function of time. After 1 hr, the conductance of LC o/w emulsions was 100.0 µS ± 9.6; for non-LC o/w emulsions, it was 61.2 µS ± 8.2. According to t-test, the conductance of LC o/w emulsions was confirmed as being significantly higher than non-LC o/w emulsions (p < 0.05). Hence, LC o/w emulsions were concluded to provide more moisturization than conventional, non-LC o/w emulsions.
Reducing TEWL: LC o/w emulsions (see Table 1d) and non-LC o/w emulsions (see Table 1b) were separately applied to skin (see Figure 10) and the TEWL of each was measured. After 2 weeks and 3 weeks of application twice daily, the TEWL of LC o/w emulsions was shown to significantly decrease, compared with non-LC o/w emulsions.
Improvement of skin condition: The skin condition of volunteers after application of the test emulsions was observed by BG dye method. The results are depicted in Figure 11. As shown, before application, multiple desquamations of corneocytes were observed and after 4 weeks of application, LC o/w emulsions reduced this multiple desquamation. However, no improvement was shown in the number of corneocytes treated with non-LC o/w emulsions. These results suggest that LC o/w emulsions normalized the epidermal differentiation process due to their higher water-retaining ability, and that the normalization contributed to the improvement of skin barrier function.
Conclusion
The present study was conducted to find an optimized approach to enhance and strengthen the skin barrier function of cosmetic formulations. This study shows that skin barrier function of formulations can be improved merely by focusing on the relationship between the structure and function of emulsion films, without the need for special active ingredients.
Utilizing a hydrogenated lecithin, PEG10-30-SS, PS and fatty alcohols, an advanced LCB was achieved that can be incorporated into LC o/w emulsions. The structure of the LCB was confirmed as a multilayer LC form, surrounding oil droplets. In addition, LC o/w emulsions were shown to maintain stability as a multilayer LC structures on the skin surface in actual applications.
LC o/w emulsions were confirmed to possess moisturizing and water loss-prevention effects, and in clinical tests, such emulsions were shown to improve the skin moisture condition of volunteers and TEWL simultaneously. Thus, this study could contribute to the development of new tools for formulating advanced skin care cosmetics.
References
1. S Nishiyama, H Komatsu and M Tanaka, A study on skin hydration with cream, J Soc Cosmet Chem Japan 16(2) 136-143 (1983)
2. L Norlen, Skin barrier Formation: The membrane folding model, J of Invest Dermatol 117(4) 823-829 (2001)
3. T Okamot, Y Matsushita, E Matsuura and M Masuda, The preparation of visible emulsion and its applications to cosmetics, J Soc Cosmet Chem Japan 39(4) 290-297 (1983)
4. T Suzuki, J Fukasawa and H Iwai, Multi-lamellar emulsion of stratum corneum lipid, J Soc Cosmet Chem Japan 27(3) 193-205 (1993)
5. Y Aoki and Y Sumida, Enhancement of moisturizing abilities of skin care products by a novel water retaining system, 22nd IFSCC congress, Edinburgh, podium presentation 38 1-8 (2002)
6. T Ochiai, H Sagitani and K Itho, Characteristics of polyglycerin copolymer type nonionic surfactants as cosmetic emulsifiers, J Soc Cosmet Chem Japan 22(3) 171-177 (1988)
7. T Suzuki, H Takei and S Yamazaki, Formation of fine three-phase emulsions by the liquid crystal emulsification method with arginine β-branched monoalkyl phosphate, J Colloid Interface Sci 129(2) 491-500 (1989)
8. T Suzuki and H Iwai, Formulation of liquid emulsions and clear gels by liquid crystal emulsion, IFSCC 9(3) (2006)
9. T Inoue, K Tsujii, K Okamoto and K Toda, Differential scanning calorimetric studies on the melting behavior of water in stratum corneum, J Invest Dermatol 86 689-693 (1986)
10. RS Summers, B Summers, P Chandar, C Feinberg, R Gursky and AV Rawlings, The effect of lipids with and without humectant on skin xerosis, J Soc Cosmet Chem 47 27-39 (1996)
11. T Suzuki, A Tsutsumi and A Ishida, Secondary droplet emulsion: Contribution of liquid crystal formation to physicochemical properties and skin moisturizing effect of cosmetic emulsion, J Soc Cosmet Chem Japan 17(1) 6-70 (1983)
12. IY Kim, CK Zhoh and HC Ryoo, Liquid crystalline technology of cosmetic industry and moisturizing effect of skin, J Soc Cosmet Sci Korea 30(2) 279-294 (2004)
13. J Levin and HI Maibach, Correlation transepidermal water loss and percutaneous absorption, Cosm Toil 120(7) 28-31 (2005)
14. R Shukla, V Bansal, M Chaudhary, A Basu, RR Bhonde and M Sastry, Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment, Langmuir 21 10644-10654 (2005)
15. DC Swartzendruber, PW Wertz, DJ Kitko, KC Madison and DT Downing, Molecular models of the intercellular lipid lamellae in mammalian stratum corneum, J Invest Dermatol 92(2) 251-257 (1989)
16. M Lynch and P Spicer, Bicontinuous liquid crystals: Cubic phase and human skin: Theory and practice, New York: Taylor & Fancis Group CRC Press (2005) 41-57