The skin is a dynamic barrier that protects the body from external sources of harm. One of the primary purposes of this barrier is to prevent infection. While the skin does provide protection from invading microorganisms, healthy skin contains a complex microbiota of normal, commensal microbial flora. An intricate balancing act exists between the skin and the wide array of microbes that constitute the normal flora. Environmental, physiological, biochemical, mechanical and immunological variables all contribute to maintain a healthy balance between the skin and its normal flora. When this balancing act is disrupted by internal or external forces, infection can occur. Infections can be caused by either normal commensal microbes or by external invading pathogens.
Colonization of the skin by normal, commensal microbes is vital to providing adequate protection from invading pathogens. Commensal microbes provide protection from invading pathogens by both direct and indirect means. Commensal microbes directly protect against invading pathogens through the excretion of antibiotics such as bacteriocins and the production of toxic metabolites. Likewise, the microbes indirectly protect through the depletion of available nutrient stores, blocking pathogen adherence and degrading toxins released by potential pathogens. It has also been found that certain commensal microbes can upregulate keratinocyte production of antimicrobial peptides.
Since establishing normal commensal flora is a key protective attribute of skin, colonization takes place immediately upon birth. Typically, the first colonization occurs as the newborn leaves the uterus and descends thru the vaginal canal.5, 6 Colonization can also begin in utero if protective membranes are ruptured prematurely and organisms are allowed access to the uterus.7 The skin of neonates born via cesarean section is typically sterile at birth since there is no exposure to the vaginal environment,8 although colonization appears to progress normally into infancy. Other sources of microbes, including handling by parents and other individuals after birth and environmental exposure, contribute to colonization of neonatal skin.2
The first and most predominant organism that colonizes the skin at birth is the aptly named Staphylococcus epidermidis. This is not surprising given the frequency that neonates are exposed to this bacterium; not only is S. epidermidis the most predominant microbe in the vagina just prior to birth, but it is also quite commonly encountered in the environment. Approximately 10–15 other species of Staphylococcus are commonly found as commensal microbes on skin.2
All commensal species of Staphylococcus are marked by the common characteristic of their inability to produce coagulase, an important enzyme in the virulence of pathogenic Staphylococcus species, such as S. aureus.2 Other commensal microbes that colonize the skin shortly after Staphylococcus spp. include members of the genera Escherichia, Bacillus, Proteus, Enterobacter, Brevibacterium, Micrococcus, Corynebacterium, Propionibacterium, Acinetobacter, Streptococcus and Malassezia.
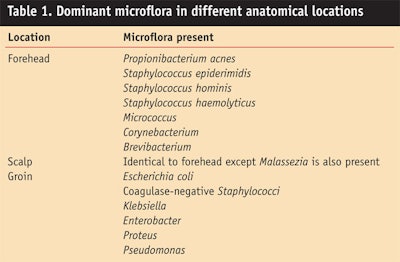
Infant Normal Flora by Anatomical Location
Distinct differences have been reported between the normal flora of infant skin and that of other age groups.9 Not all genera are ubiquitous throughout the infant body. The diversity of microbes and anatomical sites is important to understand the key maladies observed in infants. While differences have been reported for many diverse anatomical locations, the locations listed in Table 1 focus on the impact of normal flora in the pathogenesis of specific skin maladies and concerns in infants.
Infant Skin Diseases
The microbial community on and within the human body changes with growth, and the human body is influenced by its presence on a daily basis. When the delicate balance between commensal microbes and the skin is perturbed, infections and subsequent disease can develop. In this section, some common infant skin maladies and the influence of skin microbiology and physiology in their etiology are reviewed.
Impetigo: Impetigo is a superficial, bacterial infection of the skin that is primarily seen in children under the age of six. It is the third most common skin disease of infants and is characterized by a localized, inflamed and infected epidermis.10 Impetigo has two main clinical forms classified on the basis of morphology into non-bullous and bullous. Both forms are highly contagious, but measures can be taken to aid in treatment and prevention.
Although both forms of impetigo may clear slowly even without treatment, untreated disease tends to spread and persist on the skin or inanimate objects where it can act as a source of infection to others.11 Depending on the severity and number of lesions present, topical and/or oral antibiotics may be administered.10 The use of mild antiseptics to cleanse areas surrounding ruptured pustules is also recommended.11 Studies have shown that applying a prophylactic, topical antibiotic ointment to areas of mild skin trauma, such as insect bites and scratches, decreases the rate of impetigo in children.12
Because impetigo lesions are highly contagious and can spread rapidly through a family, nursery or school classroom,13 good personal hygiene and cleanliness are important in preventing transmission of the disease to others. Regular hand washing with antibacterial soap is an effective method to stop the spread of infection.13 Separation and hot water washing of clothing and bed linens helps prevent direct contact with contaminated objects that can lead to infection.12, 14 Replacement of cloth towels with disposable paper towels may also help prevent transmission.
Diaper dermatitis: Diaper dermatitis (diaper rash) accounts for more than one million clinic visits per year and is considered the most common skin disorder of infancy in the United States.15 The prevalence of this disorder, although likely underreported, ranges from 4–15% from early infancy to two years old. It most commonly occurs as early as the first few months of life.16
Common treatments of diaper dermatitis include keeping the diapered area dry and clean, applying ointments, increasing the baby’s fluid intake and giving sitz baths. During diaper change, the affected area should be cleaned with mild soaps or wipes.15 Corn starch should be avoided since it may promote Candida growth.17 In more severe cases, non-fluorinated, low-dose topical steroids and/or topical 4% sucralfate can also be used. Prescription antifungal ointment is indicated for use only in cases that have been confirmed as candidal infections.15 Disposable diapers containing super absorbent gel and breathable covers have been found to decrease the incidence of symptoms by decreasing wetness and promoting normal skin pH.16, 18
Seborrheic dermatitis: Seborrheic dermatitis (SD), or cradle cap, is a common condition of infancy seen on the scalp. SD is currently identified as an inflammatory disorder that is due to a combination of shedding skin and sebaceous gland activity. Since there is some debate over the cause, and there is no widely accepted clinical or other diagnostic criterion, there is little data on its frequency in infants.16, 19 In Australian pediatric clinics, the relative frequency of SD occurs in 4.5% of children under the age of six, although this may be underreported.19 It is most commonly seen in the first three weeks to three months of life.
Physical removal of the scales via gentle massaging with non-irritating products and daily shampooing is an effective treatment.20 The crust can also be loosened by applying slightly warmed mineral or olive oil to the affected areas. Antifungal and topical hydrocortisone treatments may be used but with caution since they are not currently recommended for use on children.16 Products containing salicylic acid should be used only with the most severe causes due to the risk of absorption.16, 20
Atopic eczema: Atopic eczema is a hereditary hypersensitivity response affecting 10–20% of the childhood population. Most cases appear from when an infant is a few months to the age of one.20 The etiology of atopic dermatitis remains unknown, although immunologic factors and subsequent allergies are thought to play a role.
Parents should be instructed to limit bathing and use lubricants and emollients to help decrease dryness and associated itching. A mild soap, bath oils and tepid water can be used during infrequent bathing.16 Soft cotton clothing should be worn to reduce itching and irritation.21 Contact with potential allergens, such as cigarette smoke and pets, should be kept to a minimum. If a food allergy is suspected, as it is in 20% of cases, those foods should be avoided until the condition is controlled.16, 22
Topical application of open wet compresses soaked in aluminum acetate can be used to soothe and suppress weeping lesions.16 In addition, topical corticosteroids can be effective treatments; however, ultra high potency corticosteroids, such as group I corticosteroids, are not recommended for use on patients younger than 12 months old due to the high risk of side effects such as dermal atrophy. Urea- and α-hydroxy lactic acid containing preparations; antipruritic agents such as antihistamines; pramoxine; caffeine; and thymic hormones are also effective.22
Emerging Treatments
The presence of microbial species adapted to the skin constitutes one of the many defenses humans have against pathogenic bacteria and fungi. Nondiscriminate removal by surface active agents or antimicrobials can destroy this beneficial ecology. It is therefore a health advantage to use a gentle, nontoxic approach to remove pathogens from the skin. The concern over use of antimicrobial agents due to their toxicity and impact on the environment is a current trend. Studies suggest that antimicrobial agents survive waste-water treatment processes and can be detected at low levels in surface waters and sediment.16, 23, 24
These concerns and others provide a strong rationale for use of alternative methods of infection control, especially with infants.
There are a number of emerging technologies that may provide adequate microbial control for normal day-to-day use products. These alternative methods include the use of technologies that enhance the removal of microbes through attractive or repulsive chemistries, the use of natural host defense molecules, and the use of pre- and probiotics.
Enhanced removal of microbes from skin: Cleaning the skin is the major benefit of personal care wipes. In the case of perineal skin, microorganisms and other fecal-derived soils present unique challenges. Microorganisms attach to the skin by multiple mechanisms,25, 26 requiring a complex removal strategy employing surfactant action, shear forces and affinity binding. A common method for removing microbes from the skin employs a surfactant combined with wiping. This approach, although practical, may be less than desirable with regard to the overall health of the skin. Cleaning products should be selected judiciously since even mild surfactants may greatly impact the integrity of the stratum corneum barrier and potentially cause skin irritation.27
The inherent negative charge of biological membranes can be used to enhance microbe removal from the skin.28 For example, negatively charged particles can enhance cleaning, provided the electrostatic forces are close enough to overcome the attraction of the microbe to the skin. It has been reported that anionic particles can displace yeast from skin.29 Additionally, positively charged wipes can also be used to enhance cleaning by attracting microbes from the skin to the wipe.
Skin cleansing products incorporating cationic compounds such as aluminum chlorohydrate30 or octadecyldimethyltrimethoxysilylpropylammonium chloride31 provide this attribute. Both of these technologies have advantages over traditional cleaning compositions because the contaminant is not merely dislodged from the skin surface, but is first dislodged then removed from the skin’s surface through interactions with the cleansing product. These methods result in a gentle and nontoxic removal of microbes from the skin.
Use of alternative control of pathogens: The human skin itself represents a physical barrier against invading pathogens.32 In addition, skin lipids such as sphingosine act as natural antimicrobials.33 Human skin also contains at least two types of antimicrobial proteins: cathelicidins and defensins.34 Cathelicidins, which appear to kill bacteria via a unique electrical charge effect, are produced by keratinocytes and are frequently found in skin wounds and sites of skin inflammation. Other defensins are created in mucous membranes and tissues lining the gut and the bronchial system.
Another antimicrobial compound found in sweat is dermcidin, which appears to be broad-spectrum in efficacy.35 Furthermore, many plants produce defensin-like proteins for control of pathogenic bacteria and fungi.36 Although it is possible to use these compounds as effective consumer antimicrobial products, antimicrobial peptides are not widely employed in the consumer market due to regulatory constraints as well as cost. Their use is anticipated to grow in the future.
Another emerging area of control employs altering the virulence of the pathogen through modulation of that organism’s physiology. Quorum sensing in bacteria is a process that can be used to control the virulence and infectious nature of bacteria.37 This process is comparable to pheromone signaling in eukaryotes as an inter-organism communication system. Quorum sensing systems regulate gene expression in bacteria in response to fluctuations in cell-population density. The quorum sensing-regulated genes tend to be important to the survival of the bacterial species at high-density conditions.38 These processes include competence, virulence, conjugation and antibiotic/bacteriocin production to control competing microorganisms.
Disruption of quorum signaling in some bacterial species, either through blockage of the signaling peptides or application of signaling peptide agonists, has been shown to modulate the processes controlled by that quorum sensing system. There are emerging actives on the market that can impact quorum sensing, hence altering the ability of the bacteria to infect
a host. Although not widely employed in commercial products, this area is ripe for use in consumer products. Quorum sensing is known to control the virulence and attachment of C. albicans to the skin. It is therefore possible to use a quorum sensing inhibtor such as farnesol to block attachment of this yeast to skin and reduce its impact on diaper rash.
Pre- and probiotics in skin products: Applications of probiotics and prebiotics have received growing attention and research funding in recent years. The United Nations Food and Agriculture Organization (FAO) and the World Health Organization (WHO) define probiotics as, “live microorganisms, which, when administered in adequate amounts, confer a health benefit to the host.”39 Prebiotics refer to nutrients that promote the growth of certain microorganisms, typically for the purpose of benefiting host health. Some formulations of probiotics and prebiotics have received more scientific scrutiny than others but there is sufficient evidence to support that this technology can alleviate certain illnesses.40
For products currently marketed that claim a pre- or probiotic ingredient, there is probably more marketing than science involved. The general population has a sense of familiarity with the use of probiotics through the consumption of yogurt and taking lactobacilli, and a general feeling that probiotics are helpful. Most products are probably using conventional lactobacilli, which has questionable value for any skin application due to the organism’s ability to survive and proliferate. However, the probiotic principle is likely to be applicable to any environment where a normal microbiota exists. Probiotic and prebiotic substances have the potential to provide a gentle and sustainable alternative to nonspecific antibacterial actives that can cause irritation and lead to possible antibiotic resistance. It has been observed that infants who were fed formula supplemented with probiotics had fewer episodes of diarrhea.41
Conclusions
The skin microflora and physiology of infants has distinct characteristics that are involved in skin maladies such as impetigo, dermatoses and eczema. Current preventive and active treatment measures have been shown to be effective; however, there are opportunities to enhance the gentleness and specificity of treatment measures. The potential risks inherent with continuous use of antibiotic and antimicrobial treatments (e.g., risk of eliminating protective, normal microflora and developing resistant organisms) warrants continuing investigation of alternative methods for skin care. This is particularly important with infant skin as its susceptibility to and consequence of infection can be greater than that of the skin of older children and adults.
References
1. R Sidbury and GL Darmstadt, Microbiology, ch 2 in: Neonatal skin: Structure and function, SB Hoath and HI Maibach, eds, New York: Marcel Dekker Inc. (2003) pp 21-46
2. M Wilson, The skin and its indigenous microbiota, ch 2 in: Microbial inhabitants of humans, M Wilson, ed, Cambridge, UK: Cambridge University Press (2005) pp 51-106
3. RR Roth and WD James, Microbiology of the skin: Resident flora, ecology, infection, J Am Acad Dermatol 20:367-390 (1989)
4. SB Hoath, Physiologic development of skin, ch 10 in: Fetal and neonatal physiology 3rd Ed., RA Polin, WW Fox and SH Abman, eds, Philadelphia, Pa., USA: Saunders (2004) pp 597-611
5. MJ Marples, The normal flora of the human skin, Br J Dermaol 81(suppl 1) 2-12 (1969)
6. I Sarkany and CC Gaylarde, Skin flora of the newborn, Lancet 1 589-590 (1967)
7. SB Winram et al, Characterization of group B Streptococcal invasion of human chorion and amnion epithelial cells in vitro, Infect Immun 66 4932-4941 (1998)
8. RA Schwartz et al, Seborrheic dermatitis: An overview, Am Fam Physician 74 125-120 (2006)
9. DA Somerville, The normal flora of the skin in different age groups, Br J Dermatol 81 248-258 (1969)
10. G Darmstadt and A Lane, Impetigo: An overview, Pediatric Derm 11(4) 293-303
11. M Sladden and G Johnston, Current options for the treatment of impetigo in children, Expert Opin Pharmacother 6(12) 2245-2256 (2005)
12. J Hirschmann, Impetigo: Etiology and therapy, Curr Slin Top Infect Dis 22 42-51 (2002)
13. G Johnston, Treatment of bullous impetigo and the staphylococcal scalded skin syndrome in infants, Expert Rev Anti-infect Ther 2(3) 439-446 (2004)
14. P Hogan, Impetigo, Aust Fam Physician 27(8) 735-736 (1998)
15. LS Nield et al, Prevention, diagnosis and management of diaper dermatitis, Clinical Pediatrics 46(6) 480-486 (2007)
16. JK Singleton, Pediatric dermatoses: Three common skin disruptions in infancy, The Nurse Practitioner 22(6) 32-50 (1997)
17. S Adalat et al., Diaper dermatitis-frequency and contributory factors in hospital attending children, Pediatr Dermato 24(5) 483-8 (2007)
18. BH Keswick et al., Diaper area skin microflora of normal children and children with atopic dermatitis, J Clin Microbiol 25 216-221 (1987)
19. P Foley et al, The frequency of common skin conditions in preschool-aged children in Australia: Seborrheic sermatitis and pityriasis capitis (Cradle Cap), Arch Dermatol 129 318-22 (2003)
20. D Orchard et al, Rashes in infants. Pitfalls and masquerades, Aust Fam Physician 30(11) 1047-51 (2001)
21. CT Cunningham et al, Formulating for children’s sensitive skin, Cosm & Toil 122 49-54 (2007)
22. S Hurwitz, Clinical Pediatric Dermatology 2nd ed., Philadelphia, Pa., USA: W.B. Saunders Co. (1993) pp 7-60
23. Anomynous, Society’s evidence on resistance to antimicrobial agents, J Pharm 259 919-921 (1997)
24. DM Dwyer, Antimicrobial resistance: Solutions for this growing public health threat. Presentations to the Hearing of the Senate Committee on Health, Education, Labor and Pensions, Subcommittee on Public Health (1999)
25. P Gilbert et al, Surface characteristics and adhesion of Escherichia coli and Staphylococcus epidermidis, J Appl Bacteriol 71 72-77 (1991)
26. M Habash and G Reid, Microbial biofilms: Their development and significance for medical device-related infections, J Clin Parmacol 39 887-898 (1999)
27. A Di Nardoet al, Sodium lauryl sulfate (SLS) induced irritant contact dermatitis: A correlation study between ceramides and in vivo parameters of irritation, Cont Derma 35 86-91 (1996)
28. NA Campbell, Biology 3rd Ed., Redwood City, Ca., USA: The Benjamin/Cummings Publishing Co., Inc. (1992)
29. US Patent 6,764,988, DW Koenig et al, Skin cleansing composition incorporating anionic particles (2004)
30. US Patent Application 20070124337, JM Villanueva et al, Bacteria binding products (2007)
31. US Patent Application 20040009141, DW Koenig et al, Skin cleansing products incorporating cationic compounds and aluminum chlorohydrate (2004)
32. PM Elias, The skin barrier as an innate immune element, Semin Immunopathol 29 3-14 (2007)
33. DJ Bibel et al, Topical sphingolipids in antisepsis and antifungal therapy, Clin Exp Dermatol 20 395-400 (1995)
34. K Yamasaki and RL Gallo, Antimicrobial peptides in human skin disease, Eur J Dermatol 18 11-21 (2008)
35. JM Schröder and J Harder, Antimicrobial skin peptides and proteins, Cell Mol Life Sci 63:469-86 (2006)
36. JH Wong et al, A review of defensins of diverse origins, Curr Protein Pept Sci 8 446-59
37. MB Miller and BL Bassler, Quorum sensing in bacteria, Annu Rev Microbiol 55 165-99 (2001)
38. NC Reading and V Sperandio, Quorum sensing: The many languages of bacteria, FEMS Microbiol Lett 254 1-11 (2006)
39. AC Ouwehand et al, Probiotics for the skin: A new area of potential application? Lett Appl Microbiol 36 327-31 (2003)
40. D Bockmühl et al, Prebiotic cosmetics: An alternative to antibacterial products, Int J Cosmet Sci 29 63-4 (2007) 41. Z Weizman et al., Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents, Pediatrics 115 5–9 (2005)