
In this edition of Comparatively Speaking, Tony O'Lenick, of Nascent Technologies Corp., turns to Ricardo Diez, Ph.D., adjunct professor at Rutgers University, to explain what crypto anionic surfactants are, as well as their utility in personal care formulations. Following are his insights.
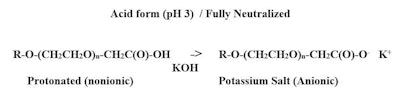
The term crypto anionic surfactant describes anionic surfactants that is capable of being anionic, nonionic or mixtures of both. The transformation from one to the other is achieved by adjusting the pH value, as shown here in Figure 1:
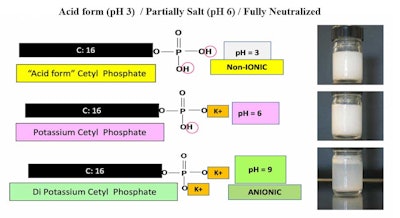
Crypto anionic surfactants are mainly in a nonionic state when the pH is below 3. At a pH of 3, the approximate concentrate of anionic and nonionic are about equal. As the pH is increased, for every one unit, the concentration of anionic material increases 10 times, as predicted by the Henderson-Hasselbalch Equation:1
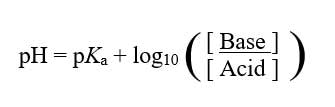
Additional examples of crypto anionic surfactants include deceth-8 carboxylate, laureth-7 citrate, PPG-5-ceteth-10 phosphate, phenol-6 EO phosphate and oleth-10 phosphate (see Figure 2).
Crypto anionic surfactants can be used to make ethanol-free perfumes. As a related patent2 states, "crypto anionic surfactants are surfactants that behave like nonionic at low pH and as anionic at high pH, and mixtures thereof in at pH values in between the two. Within the pH range of high and low, anhydrous perfumed compositions are stable and unlike conventional anionic surfactants, crypto anionic surfactants are compatible with cationic surfactants in a broad pH range."3
Figure 1 shows images of the solubility of cetyl phosphate solution at different pH values. At a high pH, foam is even visible. It is not totally clear because there is about 20% dicetyl phosphate present, which is very insoluble.
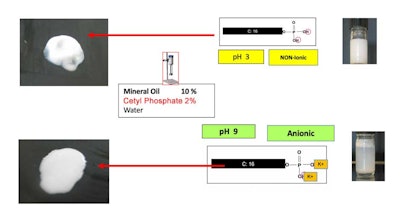
Finally, Figure 3 shows two emulsions made with mineral oil adjusting the pH of the emulsions, and therefore the pH of cetyl phosphate, to low and high pH. The difference in the emulsion is clearly obvious.
References
1. Thought Co. (accessed 2022, Mar 30). Henderson-Hasselbalch Equation and Example. Available at https://www.thoughtco.com/henderson-hasselbalch-equation-and-example-603648
2. U.S. Patent 6,803,050 (2004, Oct 12). Transparent aqueous compositions comprising hydrophobic silicone oils. Assigned to Denzer, et al.
3. U.S. Patent 9,988,591 (accessed 2022, Mar 30). Perfume compositions. Assigned to Diez, et al.










