This article briefly reviews sunscreens raising recent concern and related regulatory actions. It also proposes approaches to adapt formulas to the limitations imposed by both regulations and market trends/demands.
Sunscreens, perhaps more than other types of cosmetic products, are constantly evolving, driven by regulatory updates on one hand and changing consumer needs on the other. Several trends have recently shaped the sun care industry. For example, with the rise of K-beauty in the global market, the demand for ultra-light textures with an innovative skin feel has grown significantly.1
In addition, environmental concerns and the potential negative effects of certain chemical UV filters have shifted consumer preferences toward mineral filters including titanium dioxide and zinc oxide. Such mineral filters have also starred in trending products, e.g., those having reduced water content or in completely anhydrous bases like sticks or face powders, created for easy on-the-go application.2
Multifunctional sun care is also growing in interest to provide benefits alongside UV protection such as anti-aging or anti-spot effects, achieved by including given active ingredients. Moreover, these products may feature special pigment combinations to achieve effective color correction effects.3
At the same time, recent years have seen increased restrictions on the use of certain UV filters due to potential safety concerns. For instance, investigations into the possible endocrine-disrupting properties4 of some chemical filters and/or their aquatic toxicity5 are ongoing. This has raised consumer mistrust and, in turn, demand for products omitting these ingredients.
Here, we briefly review sunscreens that are raising recent concerns, along with related regulatory actions. We also offer approaches to retrofit and adapt formulas based on the limitations imposed by both regulations and market demand.
Sunscreen Ingredients Raising Concern
Octocrylene: The biggest storm currently raging is against octocrylene, which has been implicated in endocrine disruption.6 Although it is considered safe by the EU Scientific Committee on Consumer Safety (SCCS)7 at up to 10% in all products (except propellants, with a 9% limit), and the evidence for endocrine-disrupting effects is not sufficiently conclusive, there is increasing demand for octocrylene-free sunscreen formulations.8 In October 2023, France even submitted a proposal to restrict the placement of mixtures containing octocrylene on the market. This UV filter has also been alleged to pose environmental risks to non-target organisms in aquatic ecosystems (freshwater and seawater), including sediments, and to consumers, due to potential groundwater contamination.9
Salicylates: Another class of substances under scrutiny for potential endocrine disruption is salicylates.10 For this reason, the European Commission has reassessed ingredients such as salicylic acid and homosalate.11 New usage limits have been set for homosalate: a maximum of 7.34% in facial products only, excluding propellent spray products.12 However, this limitation is not related to the potential endocrine effects of the ingredient, rather to new repeated dose toxicity data used for the calculation of the margin of safety.
Like octocrylene, concerns over salicylates as potential endocrine disruptors lack conclusive evidence. This uncertainty extends to other ingredients such as ethylhexyl salicylate. For example, according to the European Chemicals Agency (ECHA), ethylhexyl salicylate is described as “highly toxic to aquatic life with long-lasting effects and is suspected of causing harm to unborn children.”13 While a more in-depth evaluation is warranted, in the meantime, the doubts raised about these substances have led consumers to avoid sunscreens containing salicylates.
Avobenzone: Avobenzone continues to face challenges as well. It is widely known that this UVA filter can degrade in sunlight, resulting in a loss of UV protection. Moreover, its degradation produces byproducts that may harm skin health, potentially causing irritation and allergic reactions.14 Avobenzone can be stabilized by combining it with other UV filters, particularly octocrylene, although as stated, the use of octocrylene is increasingly restricted.
Further information has been sought regarding the potential toxicity of avobenzone to aquatic life and its role as an endocrine disruptor, although environmental studies required by the ECHA for butyl methoxydibenzoylmethane (syn. avobenzone) – such as the Daphnia reproduction test and the fish early-life-stage toxicity test – have shown no toxic effects. Consequently, its current self-classification as Aquatic Chronic 4 has been revoked.15
Diethylamino hydroxybenzoyl hexyl benzoate (DHHB): Another potential up-and-coming sunscreen of concern is DHHB. German authorities found levels of the phthalate MnHexP in human urine samples, and this ingredient is banned and is listed in Annex II/1559 – the substance could also potentially be a trace of DHHB.16
This issue sparked some media attention in Germany, although authorities concluded that adverse effects were unlikely due to the low levels of MnHexP detected, compared to the tolerable daily intake (TDI).17 The matter was subsequently brought to the attention of the European Commission and other Member States. DG GROW, the EU Commission’s Directorate General for Internal Market, Industry, Entrepreneurship and SMEs, is now planning to task the SCCS with investigating whether the current use of the UV filter DHHB poses a potential risk to human health.18
Alternative Sunscreen Formulating Strategies
Considering these uncertainties, along with consumer perceptions, what strategies should formulators adopt to develop sunscreens that align with market demands, provide high UVB/UVA protection and remain sensorially appealing to consumers?
Using only mineral filters: One option is to use only mineral filters. According to the re-evaluation published by the U.S. Food and Drug Administration (FDA) in 2021,19 to date, the only two UV filters that can be considered generally recognized as safe and effective (GRASE) are titanium dioxide and zinc oxide. They reportedly are not absorbed by the skin and do not enter the bloodstream; consequently, they are not associated with potential effects as endocrine disruptors. They would also likely be safer for aquatic organisms than chemical filters.
However, there are conflicting results that their nano forms may bioaccumulate and may be associated with coral bleaching.20 Furthermore, formulating with mineral filters alone is limiting in terms of the types of products that can be created – primarily anhydrous products or w/o emulsions; the latter because w/o emulsions have a high percentage of oils that can adequately wet mineral sunscreen powders.
In o/w emulsions, beyond the difficulty of incorporating high concentrations of mineral filters, there is a risk of a gradual increase in pH due to the partial solubility of zinc oxide in water.21 This can also lead to agglomeration, with a consequent reduction in sun protection efficacy.
With mineral filters alone, it is therefore not easy to obtain textures that align with current market demands.
Omitting/replacing octocrylene: An alternative option is to formulate products excluding the UV filters of concern. The most challenging substitution is that of octocrylene, for several reasons. It is a highly effective liquid UV filter that, as stated, can be used up to 10% in all products except propellant sprays (maximum 9%). And in addition to providing UVB protection, octocrylene has excellent solubilizing properties for solid chemical filters, allowing for products with good sensory characteristics. Furthermore, it is a relatively low-cost ingredient. Thus, replacing octocrylene involves the need to introduce additional oils with high solubilizing properties, as well as other UV filters to achieve the desired SPF.
Fat-soluble replacements: If the replacement filters are fat-soluble solids such as ethylhexyl triazone, diethylhexyl butamido triazone or bis-ethylhexyloxyphenol methoxyphenyl triazine, additional solubilizing oils will be required. This creates potential stability issues and challenges in maintaining sensory appeal. For the solubilization of certain filters, particularly bis-ethylhexyloxyphenol methoxyphenyl triazine, alkanes can be used in small concentrations.22 They are also excellent from a skin-feel point of view.
Water-soluble replacements: Alternatively, octocrylene can be partly replaced by the water-soluble filter phenylbenzimidazole sulfonic acid (PBSA). It is a strong UVB absorber that can work synergistically with other UV filters even at low percentages. To be solubilized in water, it should be completely neutralized with bases (e.g., sodium hydroxide, TEA, arginine) at a pH of 7.0-7.5. The pH of the final formulation should also be in this range to avoid recrystallization during storage. This may be a limitation, considering that some ingredients are not compatible with these pH values (e.g., sodium benzoate, potassium sorbate).
Note that due to its salt characteristic, neutralized PBSA may also influence the stability of o/w emulsions that are salt sensitive. In addition, further attention must be paid to possible incompatibilities with certain polymers; particularly acrylates.
Omitting avobenzone: To exclude avobenzone yet obtain adequate protection against UVA, the rather “forced” choice falls on diethylamino hydroxybenzoyl hexyl benzoate. It is a photostable UVA filter that also provides a good boosting effect on SPF when used in combination with ethylhexyl triazone.23
Its presence in a formula, however, generally results in yellow coloration and, compared with avobenzone, an increase in the oiliness/shininess of the product. This skin feel is difficult to counteract, even with the use of sensory modifiers such as absorbent powders – especially considering the recent restrictions on the use of microplastics.24
An alternative strategy is the use of encapsulated UV filters with better photostability and reduced dermal absorption. In powder form, they may also help to improve the sensorial properties of sunscreen products.
Alternative Sunscreen Examples
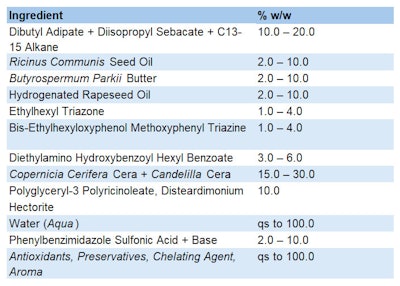
Formula 1 (see below) provides an example SPF 50 emulsion characterized by the presence of encapsulated powder filters and a particular structure that gives it the advantages of being an o/w formula with the performance of a w/o emulsion. This sunscreen features an ultra-light and quick-drying texture, matte finish and guarantees even application and high protection.
Another SPF 50 body sunscreen is shown in Formula 2 (see below). It was developed considering all the limitations of UV filters discussed while additionally omitting PEGs, ethoxylates and silicones, according to market requirements. The selected filter combination not only achieves a high protection factor, but also provides a pleasant skin feel.
From a similar base formula, it is also possible to develop an SPF 30 lipstick emulsion (see Formula 3, below). Unlike classic anhydrous sticks, this formula contains a higher percentage of water, which can be used for the delivery of water-soluble actives as well as phenylbenzimidazole sulfonic acid. It can also be prepared using only mineral filters.
Conclusions
If we were to believe every rumor, no more sunscreens would ever be formulated. Obviously this statement is intended as a provocation but in 2024, we witnessed the rise of the “anti-sunscreen” movement, particularly promoted by Gen Z on TikTok.25 Underlying this phenomenon is the belief that applying a sunscreen product is more dangerous than exposing oneself to sunlight without protection. This is because sunscreens are believed by some to be harmful to human health – even responsible for the appearance of tumors.
Formulating products according to market demands is certainly an effective strategy from a sales perspective and in some ways, quite stimulating. However, responsibility to consumers should not be overlooked, especially considering the risk of misleading them with free-from sunscreens that demonize certain UV filters without scientifically proven evidence.
References
1. Grabenhofer, R. (2024, Jul 26). 6 Cosmetics trends: Korean sunscreens, Cosmax, Kylie Cosmetics x Crumbl, "waxy" hair and more. Cosmetics & Toiletries. Available at https://tinyurl.com/yc6a9yfc.
2. Shiraz, Z. (2024, Aug 31). Sunscreen trends revealed: Innovations that will transform your summer skincare routine. Hindustan Times. Available at https://tinyurl.com/mstskcm7.
3. Doolan, K. (2024, Apr 10). 7 Sun care NPD trends to watch. Cosmetics Design Europe. Available at https://www.cosmeticsdesign-europe.com/Article/2024/04/10/7-sun-care-NPD-trends-to-watch/.
4. Gordon, J.D. and Chu, A. (2013, Aug 22). Detecting estrogenic endocrine disruptors in personal care products and supplements. Cosmetics & Toiletries. Available at https://www.cosmeticsandtoiletries.com/testing/method-process/article/21835945.
5. Grabenhofer, R. and Steinberg, D. (2018, Oct 29). [podcast] Consequences of Hawaii's sunscreen ban. Cosmetics & Toiletries. Available at https://www.cosmeticsandtoiletries.com/regulations/spf-sun/podcast/21846978.
6. L'Oreal. (Accessed 2025, Jan 8). Octocrylene: How to answer questions you are likely to be asked by your patients? Available (for download) at https://tinyurl.com/ycy6wxur.
7. SCCS (Scientific Committee on Consumer Safety). (Accessed 2025, Jan 8). Opinion on octocrylene. Available (for download) at https://tinyurl.com/55by7h53.
8. GreyB. (Accessed 2025, Jan 8). 6 Startups making BP-3 and octocrylene free cosmetics as per EU Guidelines. Available at https://www.greyb.com/blog/octocrylene-and-benzophenone-3-free-sunscreen.
9. ECHA. (Accessed 2025, Jan 8). Registry of restriction intentions until outcome. Available at https://echa.europa.eu/it/registry-of-restriction-intentions.
10. Cui, Y., He, W., Wang, Z., Yang, H., Zheng, M. and Li, Y. (2024, Sep 15). Reduced estrogenic risks of a sunscreen additive: Theoretical design and evaluation of functionally improved salicylates. Journal of Hazardous Materials. Available at https://www.sciencedirect.com/science/article/abs/pii/S0304389424019502
11. SCCS. (Accessed 2025, Jan 8). SCCS opinion on homosalate. Available at https://health.ec.europa.eu/document/download/ddf0b68f-5c47-4ace-a87f-0a0e42ebd4a9_en
12. SCCS (Scientific Committee on Consumer Safety). SCCS scientific advice on the safety of homosalate. Available at https://health.ec.europa.eu/system/files/2022-08/sccs_o_260.pdf
13. ECHA. (Accessed 2025, Jan 8). Substance infocard: 2-Ethylhexyl salicylate. Available at https://echa.europa.eu/it/substance-information/-/substanceinfo/100.003.877
14. Afonso, S., et al. (2014). Photodegradation of avobenzone: Stabilization effect of antioxidants. Journal of Photochemistry and Photobiology B: Biology, 140 36–40.
15. ECHA. (Accessed 2025, Jan 8). 1-[4-(1,1-dimethylethyl)phenyl]-3-(4-methoxyphenyl)propane-1,3-dione (substance information). Available at https://echa.europa.eu/it/registration-dossier/-/registered-dossier/14835/6/1
16. Food Packaging Forum. (2024, Feb 28). Unexpected SVHC phthalate metabolite found in humans, including children. Available at https://foodpackagingforum.org/news/unexpected-svhc-phthalate-metabolite-found-in-humans-including-children
17. German Federal Institute for Risk Management (BfR). (Accessed 2025, Jan 8). Mono-n-hexyl phthalate: Exposure estimation and assessment of health risks based on levels found in human urine samples. Available at https://tinyurl.com/2ummbtu8
18. CTPA. (2024, Oct 7). Important: UV filter DHHB - Call for interest. Available at https://www.ctpa.org.uk/news/important-uv-filter-dhhb-call-for-interest-7971
19. FDA. (2021, Sep 24). Proposed Order (OTC000008): Amending over-the-counter (OTC) Monograph M020: Sunscreen drug products for OTC human use. Available at https://tinyurl.com/4wr6b5md
20. Corinaldesi, C. (208). Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Science of the Total Environment, 637–638, 1279–1285.
21. Carlotti, M.E., et al. (2004). Study on the release properties and stability of o/w emulsions containing salicylic acid and zinc oxide. Journal of Drug Delivery Science and Technology, 14(2) 119-126.
22. Herzog, B., et al. (2022). Insights into sunscreen UV absorber solubility from theory and experiment. IFSCC Magazine, 25(2).
23. Lionetti, N. and Rigano, L. (2017). The new sunscreens among formulation strategy, stability issues, changing norms, safety and efficacy evaluations. Cosmetics, 4, 15.
24. European Union. (2023, Sep 25). Commission Regulation (EU) 2023/2055 of 25 September 2023 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) as regards synthetic polymer microparticles. Available at https://eur-lex.europa.eu/eli/reg/2023/2055/oj/eng.
25. Lee, B.Y. (2024, Jun 13). Anti-sunscreen movement spreads on TikTok. Here are issues with this trend. Forbes. Available at https://www.forbes.com/sites/brucelee/2024/06/13/anti-sunscreen-movement-on-tiktok-here-are-issues-with-this-trend/