Several publications document the benefit of electric fields in wound healing1-5 and on collagen deposition.2, 6-8 Specifically, an in vitro electric stimulation study on human dermal fibroblasts showed that the bioelectric field appears to play an important role in controlling human fibroblast activity by either significantly increasing or decreasing the gene expression of over 400 transcripts, including activity within specific cellular signaling pathways such as TGF-β, G-proteins and inhibition of apoptosis.9 Since transepithelial electrical potential in populations ages 65-80 is approximately 50% less than that in populations ages 19-29,10 it is also hypothesized that externally applied electrical currents may provide beneficial effects against skin aging signs.
In relation, the first signs of skin aging often occur in the thin, fragile periorbital skin in the form of fine lines and wrinkles, dark circles, bags under the eyes and puffiness and laxity in both the upper and lower eyelids.11 Combining these observations, the authors sought to develop an effective product to treat the signs of aging in the periorbital area based upon the concept of biomimetic electricity. A zinc and copper (ZnCu) complex-containing product was designed to meet this goal. Considering that the current gold standard cosmetic treatments for photo-damaged skin are based on tretinoin or retinol, with rather poor skin tolerance, the current article describes a clinical study designed to evaluate safety as well as efficacy of this product.
Zinc and Copper Complex
As noted, a zinc and copper (ZnCu) complex was developed to deliver low level electrical stimulation in topical anti-aging applications. The ZnCu complex is pure elemental zinc particles of several micrometers in size partially coated with elemental copper via a proprietary process, thus making the particles essentially miniature galvanic couples. When they come into contact with an electrically conductive formulation, during application to the skin, several hundred millivolts of electrical potential are created. The benefit of the electrical potential to improve the aged skin is the subject of this clinical study. Since both zinc and copper are essential minerals, the ionic by-products from the ZnCu complex are safe and beneficial in the skin care product.
This complex has been shown to safely impart beneficial physiological and clinical effects to skin.11-17 Several previous in vitro, ex vivo and clinical studies have documented its benefits, including the ability to reduce inflammatory response and UV-induced damage in human skin equivalents,17 and reduce melanin content and TYR mRNA in cultured human skin explants.15 In addition, it has been shown to increase the elastin fiber network and elastin and collagen expression in full thickness human skin explants.14
Clinically, the ZnCu complex improved several signs of aging in four efficacy trials, including one comparing a 12-week treatment with a placebo for overall appearance, under-eye bags and wrinkles, cheek wrinkles, pigmentation, radiance, fine lines and global lifting/firming in 94 subjects.12 Another assessed fine lines and wrinkles, tone, pigmentation, texture and smoothness in a 31-subject baseline-controlled study with a 4-week treatment combining the ZnCu complex with a moisturizing lotion.25
Similarly, the effects of the complex on under-eye dark circles and bags, skin radiance and overall photo-damage were tested in an 8-week, placebo-controlled, double-blind study in 123 women having mild to moderate photo-damaged skin.12 Also, skin firming and lifting, and decreases in laxity and overall signs of photo-damage were assessed in a double-blind study of 32 subjects.16 In all clinical trials, the onset of positive effects was fast for several parameters, with continued enhancement throughout the treatment; for example as early as week two and up to week 12, in one study.12
Material and Methods
The study reported here was the first to be conducted on European subjects. Its results are consistent with those previously reported for the ZnCu complex and are grounded on both subjective and objective methodologies extensively published in the literature. The non-interventional, open-label, baseline-controlled clinical study described here included a 30-day washout period and eight week treatment period conducted according to the Helsinki declaration and under the control of a dermatologist who approved the protocol and reviewed the results.
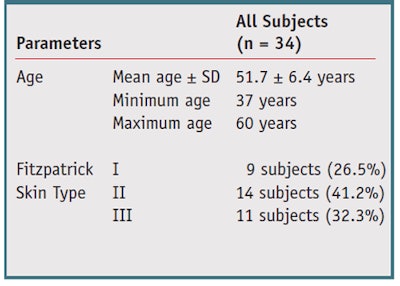
Thirty-four healthy Caucasian female subjects with Fitzpatrick Skin Type I to IV, ages 35 years and older and without any skin conditions, were selected from some 7,000 volunteers and signed an informed consent before participation. No makeup, scrubs or UV exposure via the sun or sunbeds were allowed during the study. The test protocol involved a two-step process whereby subjects applied a ZnCu complex-containing product followed by an o/w moisturizing cream in order to activate the ZnCu complex. The study personnel supervised the first-time application, during which subjects first washed their faces with their usual facial cleansers, then applied to the eye area the active followed by the moisturizer based on each individual’s typical habits and usage amounts.
The test complex plus the cream were applied each morning for eight weeks. At each study visit, subjects abstained from wearing any cosmetic in the morning. They rested quietly for ≥ 15 min, acclimating to ambient temperature (20 ± 2°C) and relative humidity (50 ± 5%) conditions. The following evaluations were conducted at each of the four study visits at baseline (T0), immediately after the first application (Timm), and at weeks 1, 4 and 8 (T1, T4 and T8).
Clinical grading18, 19 was conducted by an expert grader using 0-10 visual analog scales, where 0 = none and 10 = very severe/important.
Self-assessment questionnaires: While looking in a mirror, subjects evaluated their skin appearance and alteration/repair and rated it on a 5-point scale, where 1 = completely agree and 5 = completely disagree.
Sitting, digital standardized 2-D photographs20, 21 were taken using two different filters/lighting condition set-ups, i.e., parallel-polarized and visible. For each set-up, three different facial angles also were captured: center, 45 degrees positive (left) and 45 degrees negative (right). The distance from the camera sensor to the chin rest center was standardized at 13.5 in.
A quantitative evaluation of change in cutaneous relief22-24 was performed using skin surface replicas made with a silicon paste spread over the crow’s feet area. These replicas were processed with an image analyzer using software.
Safety also was evaluated by study technicians, who recording adverse skin events by start date, nature, intensity, localization, duration and withdrawal justification. In addition, subjects completed a self-evaluation tolerance questionnaire.
Statistical Methods
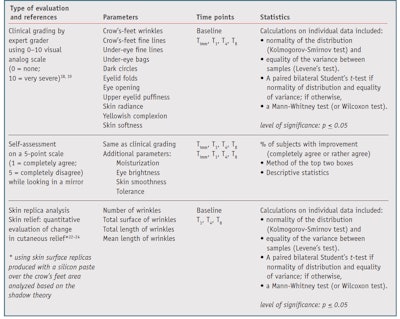
The efficacy parameters and statistical analyses for the study are listed in Table 1. For clinical grading and skin replicas, mean values, standard error to the mean (SEM) and percent changes from baseline were calculated for each measured parameter at each time point. Statistical analysis was performed using software, and self-assessment questionnaire responses were tabulated by score. Results were summarized using the “top two boxes” calculation. Subjects who did not rate a parameter at a time point were removed from the percentages.
Results
Population: Thirty-two female subjects completed the study. Their demographic information and distribution of Fitzpatrick skin types are summarized in Table 2. In comparison with baseline, the test product provided statistically significant improvements at all time points in all parameters (p ≤ 0.05), except for at the eye opening (see Figure 1, Figure 2 and Figure 3). These effects began as soon as after the first application, or after one week of product use (data not shown). The decrease in crow’s feet wrinkles and fine lines, infraorbital fine lines and eyelid folds was maintained throughout the eight weeks of treatment. Dark circles and eyelid puffiness also were reduced and continued to lessen through the duration of treatment, reaching 70% at T8. All skin complexion parameters were greatly enhanced, reaching 50% improvement and up to 80% at T8. Examples of clinical reductions in wrinkles and sagging are shown in Figure 4 and Figure 5.
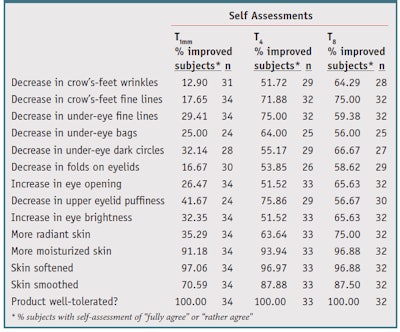
Self-assessment: At T4 and T8, 59% to 75% of subjects noted a decrease in crow’s feet wrinkles and under-eye fine lines (see Table 3). Only crow’s feet wrinkles at T4 did not reach the selected 55% threshold. Dark circles and eyelid puffiness were also greatly reduced, as judged by 55% (T4) and 66% (T8) of subjects, and 76% (T4) and 57% (T8) of subjects, respectively. At T4 and T8, 64% to 97% of subjects observed an improvement in all four skin complexion parameters. More than 90% agreed the product moisturized the skin and promoted softness after the first application and throughout the study. All subjects showed an excellent tolerance to the tested products at all time points.
Skin replicas: At the crow’s feet site, the test product provided stable, statistically significant reductions in wrinkle total surface and mean length over time, with respective decreases at T4 and T8 of 28-39% and 13-16% over baseline (see Figure 6).
Discussion and Conclusion
The ZnCu complex tested in the current study generates biomimetic electricity at a physiological level, which stimulates skin physiology. The electric potential of the ZnCu complex was recorded in vivo by a bioelectric field imagera, which noninvasively measured the current produced when the ZnCu complex was applied to the skin.27
Safety
Clinical safety was assessed through a series of tests including human repeat insult patch tests on Caucasian and Asian populations, human cumulative irritation, phototoxicity, photoallergy and clinical evaluation of safety and tolerance. Safety studies involving over 1,000 healthy subjects showed that none of the topical compositions, at any of the tested concentrations, induced dermal sensitization. In addition, the ZnCu complex, in comparison with placebo, did not induce ocular irritation in a human corneal modelb, and it was mild on skin and around the delicate eye area.13 Clinical studies also showed the complex to be well-tolerated,12, 16 to not induce adverse events,11 and to be mild and gentle to the skin.25
Although cosmetics are not required to undergo the same rigorous testing as drugs prior to approval, more recently, cosmeceutical companies have founded their claims on improved clinical data and stronger methodologies26 using double-blinded, placebo- or active-controlled design; validated scales for clinical grading by experimented investigators and for self-assessments; and diverse objective measures to evaluate outcomes, i.e., photography, skin replica methods, skin elasticity or moisture content instruments. In the described study, a placebo control was judged unnecessary since three convincing placebo-controlled studies had already been conducted.
Conclusion
In conclusion, both subjective and objective assessments demonstrated significant decreases in facial photodamage signs during an eight-week treatment with the ZnCu complex. Clinical grading and self-assessments demonstrated statistically significant improvements in crow’s feet fine lines and wrinkles, under-eye fine lines and bags, eyelid puffiness and dark circles, and skin texture—i.e., skin radiance and tone. Objective methods showed a reduction in total wrinkles surface and mean length in skin replicas. In this sensitive and fragile area, the product also was well-tolerated throughout the 8-week treatment, with no adverse events. Combined with existing data, this study suggests the elemental ZnCu complex is an interesting treatment option for anti-aging treatments in the eye area.
Acknowledgments: The authors wish to thank and acknowledge the following employees from Johnson & Johnson: Jeannette Chantalat, Elizabeth Bruning, Ying Sun and Jue-Chen Liu, for providing scientific information on previous studies performed with the mineral complex and Agnès A. Bouvet, MD, for writing the publication.
References
Send e-mail to [email protected].
- J Dube et al, Restoration of the transepithelial potential within tissueengineered human skin in vitro and during the wound healing process in vivo, Tissue Eng Part A 16(10) 3055–3063 (2010)
- A Huttenlocher and AR Horwitz, Wound healing with electric potential, N Engl J Med 356 303–304 (1987)
- CD McCaig et al, Controlling cell behavior electrically: Current views and future potential, Physiol Rev 85 943–978 (2005)
- ET Wang and M Zhao, Regulation of tissue repair and regeneration by electric fields, Chin J Traumatol 13 55–61 (2010)
- M Zhao et al, Electrical signals control wound healing through phosphatidylinositol- 3-OH kinase-gamma and PTEN, Nature 442 457–460 (2006)
- BY Lee et al, Ultra-low microcurrent therapy: A novel approach for treatment of chronic resistant wounds, Adv Ther 24 1202–1209 (2007)
- R Nuccitelli et al, Imaging the electric field associated with mouse and human skin wounds, Wound Repair Regen, 16 432–441 (2008)
- JC Ojingwa and RR Isseroff, Electrical stimulation of wound healing, J Invest Dermatol 121 1–12 (2003)
- J Jennings, D Chen and D Feldman, Transcriptional response of dermal fibroblasts in direct current electric fields, Bioelectromagnetics 29 394–405 (2008)
- R Nuccitelli et al, The electric field near human skin wounds declines with age and provides a non-invasive indicator of wound healing, Wound Repair and Regeneration19 645–655 (2011)
- J Chantalat et al, Biomimetic signaling technology generated by a bimineral complex demonstrates efficacy in reducing clinical photo-aging in a 12-week placebo-controlled study, J Am Acad Dermatol 62 AB6 (2010)
- J Chantalat et al, Biomimetic signaling technology generated by a bimineral complex reduces signs of photo-aging in the eye area, J Am Acad Dermatol 62 AB60 (2010)
- J Chantalat et al, Safety and tolerability of a topical bi-mineral complex that generates biomimetic signals, J Am Acad Dermatol 62 AB59 (2010)
- N Chen et al, Biomimetic electricity generated by an elemental bi-mineral complex increases extracellular matrix production in human skin explants, J Am Acad Dermatol 62 AB58 (2010)
- N Chen et al, Biomimetic electricity generated by an elemental bi-mineral complex inhibits melanogenesis via suppressing tyrosinase expression, J Am Acad Dermatol 62 AB59 (2010)
- S Hornby et al, Facial moisturizer featuring biomimetic signaling technology from a bi-mineral complex reduces skin laxity, J Am Acad Dermatol 62 AB60 (2010)
- P Lyte et al, Biomimetic electricity generated by an elemental bi-mineral complex produces anti-inflammatory activity, J Am Acad Dermatol 62 AB58 (2010)
- DG Land and R Shepherd, Scaling and ranking methods, in Sensory Analysis of Foods, JR Piggott, eds, Elsevier Science Publishers: Essex (1988) pp 155–186
- IFSCC Monograph Number 1: Principles of product evaluation: Objective sensory methods general introduction to product evaluation (1987)
- D Ratner, CO Thomas and D Bickers, The uses of digital photography in dermatology, J Am Acad Dermatol 41 749–756 (1999)
- F Kaliyadan, Digital photography for patient counseling in dermatology—A study, J Eur Acad Dermatol Venereol 22 1356–1358 (2008)
- TW Fischer, W Wigger-Alberti and P Elsner, Direct and non-direct measurement techniques for analysis of skin surface topography, Skin Pharmacol Appl Skin Physiol 12 1–11 (1999)
- J Gassmueller, A Kecskes and P Jahn, Stylus method for skin surface contour measurement, in Handbook of Non-invasive Methods and the Skin, J Serup, GB Jemec, GL Grove, eds, CRC Press, Boca Raton, FL, USA (2006) pp 163–168
- GL Grove, MJ Grove and JJ Leyden, Optical profilometry: An objective method for quantification of facial wrinkles, J Am Acad Dermatol 21 631–637 (1989)
- D Miller, W Wallo and Y Appa, Clinical improvements in facial photoaging with topical application of a biomimetic mineral complex and naturals extract formulation, J Am Acad Dermatol 62 AB63 (2010)
- S Bruce, Cosmeceuticals for the attenuation of extrinsic and intrinsic dermal aging, J Drugs Dermatol 7 s17–22 (2008)
- S Kaur et al, Galvanic zinc-copper microparticles produce electrical stimulation that reduces the inflammatory and immune responses in skin, Arch Dermatol Res 303, 551–562 (2011)