Editor's note: Hear more on this subject in our exclusive Author Commentary podcast.
Hair colorants are in high demand. According to a recent survey, the hair color market will continue to grow and its compound annual growth rate (CAGR) could reach 21% by 2021.1 As such, many manufacturers have introduced their own brands and rendered various claims to gain a competitive edge and differentiate their products from those of competitors. Although such claims should inform consumers about a product’s benefits, they also can be misinterpreted and misleading, causing confusion about well-established and effective ingredients.
Hair dyes provide a prime example. Product claims such as ammonia-free may be stating a fact—the omission of ammonia—but they also give consumers a negative impression of ammonia. What’s more, such claims may be irrelevant; perhaps ammonia was simply the best option for a given application.
The present article touches on misconceptions about ammonia versus monoethanolamine-based hair dyes. It also analyzes facts about these ingredients and their mechanisms, to arm formulators with both the consumer perspective and scientific rationale to choose the best ingredient for the hair dyes they develop.
Perceptions of Ammonia vs. Monoethanolamine
Since permanent hair colorants containing ammonia (NH3) have a pungent odor, hair color users believe they must be harsh on hair. And indeed, the oxidation/coupling reactions that occur during permanent dye use cause dramatic changes in hair—and damage can be the result. In response, some formulators have replaced ammonia with monoethanolamine (MEA), as it functions similarly and keeps the pH of the formula on the alkaline side. This, in turn, ensures the hair dye composition functions properly.
However, this ingredient is not an exact replacement, and offers its own benefits and drawbacks. So before making formulating decisions, it is important to consider some facts about not just ammonia but also MEA; as these two formulating options1–3 are more complicated than they appear.
First, ammonia, also known as ammonium hydroxide (NH4OH), when it is dissolved in water, is included on the U.S. Food and Drug Administration’s (FDA) list of direct food substances and is generally recognized as safe (GRAS).4 Indeed, ammonia is actually used as a food additive in baked goods, cheeses, chocolates, puddings, etc.5
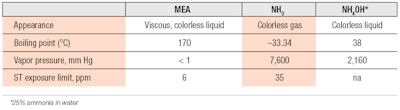
Comparisons can also be made of the physical properties of ammonia versus MEA, as reported in the literature (see Table 1). From the data, it is apparent that MEA is a liquid at room temperature and does not evaporate readily since it has low vapor pressure. On the other hand, ammonia exists as gas at ambient temperature; and although present as NH4OH in colorant compositions, ammonia still has a strong tendency to vaporize from an open surface.
Hence, if a hair colorant product is not rinsed thoroughly from the hair after treatment, one can be assured the ammonia residue will vaporize completely within a short period of time. Any MEA residue, on the other hand, would likely remain on the hair or scalp until the next hair wash. In such a situation, not only would MEA cause the hair to retain an amine smell and potentially become more damaging to hair, it may also induce skin irritation, depending on the amount of MEA residue.
Concerning the effects of these two alkalis on hair itself, Bailey et al.9 conducted a series of measurements to examine them. The researchers demonstrated that, everything being equal, in the most extreme case, the MEA-based formula caused 85% more damage to hair protein, as measured by protein loss. SEM also revealed more cuticle stripping than the product containing ammonia.
The fact that some consumers believe ammonia-based permanent hair color causes serious damage to hair may also be a product of history. They may not be aware that significant improvements have been made in hair colorant formulas to reduce damage during the dyeing process.
However, if MEA-based colorants have no advantages over ammonia-based products—other than odor—why are they on market? The fact is, in spite of the negative factors described above, MEA-based colorants enable a different level of hair color permanence and vibrancy. Furthermore, consumers are using hair color for different purposes.
Gently Does It
Not all consumers use hair dyes to dramatically revamp their appearance. A good portion uses colorants just to cover their roots or gray hairs, and many of these individuals have expressed concern over the damage that re-coloring new hair growth (see Figure 1) every eight weeks can cause. To address these concerns, a “modified” permanent hair color product employing MEA was introduced to the market.
Here, it is important to differentiate between the performance of MEA-based and ammonia-based colorant products. Ammonia-based permanent colors are used to lighten the natural pigment of hair while at the same time depositing new color to the fiber. Indeed, it has been demonstrated10 that ammonia is essential to bleaching hair’s natural melanin pigment. With ammonia, the resulting color can therefore be lighter than the original hair color before dyeing, and the new color usually lasts for up to eight weeks.
On the other hand, MEA-based formulas only allow users to keep the same color of their hair or go darker. Penetration into the hair cortex is also inferior to ammonia,2 causing the color to be less color-fast.
Formulators then cleverly replaced ammonia in permanent color formulas with MEA, which therefore only act as alkaline agents to “catalyze” the color formation without lightening capability. This allowed formulators to minimize the malodor of ammonia and ease “the damage concern” in consumers’ minds at the same time. To further enhance this “less-damaging” concept, formulators also decreased the concentration of hydrogen peroxide in the developer portion of the product. Consumers certainly appreciated these gentler product concepts, especially for already-damaged hair.
Despite some reported negative effects, MEA-based colorants enable a different level of hair color permanence and vibrancy.
Oxidation Dye Precursor Concerns
Another negative perception of permanent hair colorants is that they contain synthetic (“chemical”) dye precursors, which are believed to negatively impact one’s health, or even cause cancer. For example, Rojanapo et al.11 argued that p-phenylenediamine (PPD) can be oxidized to form a mutagenic substance in hair dye. However, results from numerous studies and research teams were unable to confirm this speculation.12
In other studies, PPD used alone was associated to dermatitis in some individuals.13 It was postulated that the actual irritant in this case was an oxidized species of PPD known as p-benzoquinone diimine.14 In relation, Gibson et al.15 found that PPD did not induce allergic contact dermatitis, although T-cell proliferative responses were detected from the oxidation product Bandrowski’s Base. The mechanism of color formation from PPD and subsequent production of both p-benzoquinone diamine and Bandrowski’s Base are illustrated in Figure 2.
It is important to understand the reaction kinetics of what PPD undergoes during the dyeing process. In the presence of hydrogen peroxide—the most common oxidizing agent used in permanent hair color, PPD can be steadily converted to p-benzoquinonediimine, as indicated in the first step in Figure 2. The reaction rate of this step has clearly been demonstrated as the slowest in the whole color formation process.16–18
In the presence of a nucleophilic coupler such as m-aminophenol (also shown in Figure 2), p-benzoquinonediimine is quickly captured to form a dimeric molecule with the rate constant k3, which is subsequently oxidized further to generate the colored indoaniline dye molecule. This conclusion is supported by the result that when two couplers are present in the reaction solution together with p-phenylenediamine, the product ratio of the two final dye molecules is in accord with the ratio of the two corresponding rate constants of the two coupling reaction k3 values.19
Furthermore, there is ample evidence showing that Bandrowski’s Base does not form during the dyeing process in current formulas.20, 21 Based on these results, one must conclude that permanent hair color or Level 3 products should be safe to use.
That said, one cannot rule out the possibility that a trace amount of oxidized PPD could exist in the pool of the dyeing composition and eventually lead to the formation of a skin sensitizer. Perhaps this is why an occasional news report describes a consumer’s allergic reaction when dyeing his/her hair. This is also why permanent hair dye labels always advise consumers to perform a pre-dyeing patch test, to ensure the user is not allergic to the product.
To circumvent potential reactions, manufacturers take additional precautionary steps: one in their choice of dye intermediates, and the other in consumer methods of application. Regarding the former, manufacturers have been actively searching for alternatives to PPD. For example, new molecules making their way to the market include 2,5-diaminotoluene,22 2-hydroxyethyl-p-phenylenediamine,23 2-methoxymethyl-p-phenylenediamine24 and 2,4,5,6-tetraaminopyrimidine.25
All of these are reported to demonstrate lower skin sensitization potential than their parent PPD compound. One drawback of these compounds is that they are not as effective in coloring hair as PPD.18 Furthermore, formula stability and/or compatibility is an additional consideration.
For this reason, other manufacturers take the latter approach. Many retail hair colors can now be applied using a comb-type applicator rather than the conventional plastic squeeze bottle with a pointed nozzle. This helps minimize contact between the dye composition and scalp skin, reducing the possibility of allergic hair dye reactions.
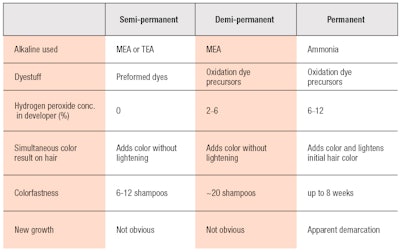
Semi-permanent colorants use dyestuffs having narrow absorption bands in the visible light spectrum, which translate to brighter colors than most permanent colorants.
Customized Color and Coverage
In contrast to ammonium-based and MEA-based colors, semi-permanent colorants do not utilize hydrogen peroxide or ammonia, or undergo oxidation/coupling reactions during the dyeing process, and are another common product to cover gray hairs. Semi-permanent colorants are gentler to hair and cause little to no damage. They only add color to hair via pre-formed dyes that diffuse inside the hair fiber. Semi-permanent colorants, in particular, use dyestuffs having narrow absorption bands in the visible light spectrum, which translate to brighter colors than most permanent colorants.
Due to this deposition mechanism, MEA-based demi-permanent and semi-permanent products are washed out more steadily than permanent colors. Demi-permanent products typically last 20 or more shampoos—which has the benefit of making root regrowth less noticeable.26 Semi-permanent colors are similar to demi-permanent ones, but designed to wash out faster; i.e., in six to 12 shampoos. Consumers who use these dyes typically do not expect the color to be long-lasting. The challenge, here, is for formulators to maintain the tonality of color on hair while it is gradually fading away upon repeated shampooing.
Subsequently, ammonia-based products were labeled as level 3 or permanent hair colorants; MEA-based products were labeled as level 2 or demi-permanent products; and semi-permanent products secure the level 1 label.27 Table 2 provides a brief summary and comparison of these three levels of hair coloring.
It should be noted that in certain parts of the world, MEA-based demi-permanent color products are known as semi-permanent colorants. Yet, they still utilize same dyestuffs and technologies as the demi-permanent colorants described in this article. There are some MEA-based permanent colorants that can lighten and color simultaneously, but these include higher amounts of both MEA and hydrogen peroxide, so the potential for irritation and hair damage is consequently higher.
The fact that demi-permanent hair colors are not permanent also seems to suit a particular market segment of customers simply wanting to “test the waters” before they commit to permanent color. Many are complacent with the product properties and characteristics of this Level 2 color.
Semi-permanent dyes also tend to be the product of choice for hair that is freshly bleached or straightened, to avoid further disruption of the internal hair structure. And since hair becomes more porous from bleaching or straightening, it is easier for the semi-permanent dye molecules to diffuse deeply inside the hair and withstand shampooing.
Needless to say, a wide range of after-treatment ingredients are designed to prevent or retard the hair color from washing away. Light-fastness is another challenge; in relation, Corbett observed that ortho-substituted and 2,5-disubstituted nitro benzenes have the best stability to light.28 Sunscreens can also be implemented; however, they must be deposited in a thick layer to trim the intensity of incident light by even only 50%, according to Beer’s Law. Hence, finding a way to photochemically stabilize the dye molecules themselves in hair remains an area for further exploration.
MEA-based semi-permanent products are washed out more steadily, which makes root regrowth less noticeable.
Natural Hair Dye Preferences
Products claiming to be environmentally sustainable, naturally derived and free from “chemicals” have gained much attention in the consumer market. And like other product consumers, many hair dye users share the opinion that a hair dye employing solely natural or organic ingredients should be kind to hair. Such hair colorants are easy to find, and bear claims for water bases or natural plant and mineral ingredients.
Natural hair dyes are usually temporary to semi-permanent in their duration. The degree of colorfastness is inferior to demi-permanent or permanent colors since these products do not contain peroxide or alkaline amines. As such, they are, indeed, usually milder to hair and less likely to cause damage to hair fibers.
An extensive discussion is under way about how to convert these natural dyes so they remain permanently on hair.29 This would not easily be accomplished and would require lengthy preparation time and effort to make it a reality. Additionally, these types of colorants do not have the capability to lighten the natural color of hair; they are deposition-only type products.
The most common natural dyes used in these products include henna, coffee, black and chamomile tea, carrot and beet juice, walnut shell, etc. Among these, products containing henna dyes are particularly popular for their simultaneous coloring and conditioning effects. It is worth pointing out there is a wide range of henna colors for both hair and skin. Henna hair colors include blonde, brown and black; however, henna plants only contain Lawson as the major coloring ingredient, which is responsible for imparting orange-red color on hair.
Chemicals, metallic salts or other vegetable dyes are sometimes added to henna powder to produce colors other than red, and these mixes are referred to as compound hennas. Interestingly, it is also not uncommon to find PPD on a henna hair dye product ingredient list.30
Another aspect to consider regarding henna hair dye is the time required to prepare the dye paste and color the hair.31 This is a much longer process than using a commercial Level 1, 2 or 3 colorant products. Lastly, colorfastness and color rub-off are additional drawbacks to consider.
Taking “natural” a step further, a recent survey showed32 there is high global demand for organic cosmetics, and this market is expected to grow 10% between 2017 and 2022. This raises some important points regarding hair dyes.
First, in order for cosmetics and personal care products to bear some sort of “organic” labeling on the U.S. market, the minimum requirement is for the total composition, excluding water and salt, to contain at least 70% organic ingredients.33 Labeling rules become more complicated from there, limiting the number of additional synthetic materials manufacturers can include in their formulas. Unfortunately, for consumers seeking hair colorants with a “100% organic” stamp on the label, the only ones they will find are likely to demonstrate poor dyeing capability, as there are not many natural and functional dyestuffs yet available.
The colorfastness of natural hair dyes is inferior to permanent colorants since they do not contain peroxide or alkaline amides. As such, they are milder to hair.
Conclusion
There are numerous hair color brands in professional salons and the retail market. To differentiate from one another, they are formulated differently to create their own unique properties. Some may be more conditioning to hair, impart a nicer fragrance to hide undesired odor, contain sunscreen to enhance the photostability of the dyes, etc.
While every product development department is busy assembling its own ingredients to generate yet another unique formula to meet the marketing need, there must be an additional intelligent team of scientists working diligently to address the shortcomings of the current products in the market and to expand the horizon of hair color technology.
Acknowledgements: The author would like to thank Mark Chang, of ChemEagle Trading Co. in Taiwan, for his suggestion and encouragement to write this manuscript.
References
All websites accessed on Jan. 9, 2018.
- technavio.com/report/global-novelty-hair-color-market
- amcojoicoiso.com/j64815_kcd_0410_measheet.pdf
- https://organichaircolorreview.wordpress.com/2013/01/03/mea
- http://blog.lavantgarde.com/?p=68
- fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/default.htm
- https://pubchem.ncbi.nlm.nih.gov/compound/ammonium_hydroxide
- https://wcam.engr.wisc.edu/Public/Safety/MSDS/Ammonium%20hydroxide.pdf
- https://en.wikipedia.org/wiki/Ethanolamine
- https://www.cdc.gov/niosh/npg/npgd0256.html
- youbeauty.com/beauty/demi-permanent-hair-color
- P Prem, KJ Dube, SA Madison and J Bartolone, New insights into the physicochemical effects of ammonia/peroxide bleaching of hair and Sepia melanins, J Cosmet Sci 54 395–410 (2003)
- W Rojanapo, P Kuradinun, A Tepsuwan, S Chutunataewin and M Tanyakaset, Carcinogenicity of an oxidation product of p-phenylenediamine, Carcinogenesis 7(12) 1997–2002 (1986)
- B Takkouche, M Etminan and A Montes-Martinez, Personal use of hair dyes and risk of cancer: A meta-analysis, JAMA 293 2516–2525 (2005)
- JP Thyssen, JML White, Epidemiological data on consumer allergy to p-phenylenediamine (PPD), Cont Derm 59(6) 327–343 (2008)
- L Schwartz, The diagnosis of cosmetic dermatitis, JSCC 1 46–53 (1947)
- A Gibson, S Kim, F Lee, BK Park and DJ Naisbitt, Characterization of primary human T-cell responses to p-phenylenediamine and Bandrowski’s base, poster presentation, 6th Drug Hypersensitivity Meeting (DHM6), Bern, Switzerland (Apr 9–12, 2014)
- Y Feng and A Chan, Kinetics and mechanism of indo-aniline dye formation in aqueous peroxide solution, JSCC 45 299–308 (1994)
- ZS Pillai and PV Kamat, Spectroelectrochemistry of aromatic amine oxidation: An insight into the indo dye formation, Res Chem Intermed 31 103–112 (2005)
- JF Corbett, The role of meta difunctional benzene derivatives in oxidative hair dyeing. I. Reaction with p-diamines, JSCC 24 `02–`34 (1973)
- K Brown, Hair Colorants, JSCC 33 375-383 (1982)
- HH Tucker, Hair coloring with oxidation dye intermediates, JSCC 18 609–628 (1967)
- M Altman and MM Rieger, The function of Bandrowski’s Base in hair dyeing, JSCC 19 141–148 (1968)
- A Scheman, C Cha and M Bhinder, Alternative hair-dye products for persons allergic to p-phenylenediamine, Dermatitis 22(4) 189–192 (2011)
- U.S. Pat 4,840,639, Agent for dyeing hair, H Husemeyer, H Mager and E Konrad, assigned to Wella (Jun 20, 1989)
- U.S. Pat 6,503,282, Means and method for dying (sic) keratinic fibers, H Braun, assigned to Wella (Jan 7, 2003)
- http://science.howstuffworks.com/innovation/everyday-innovations/hair-coloring3.htm
- AD Bailey, G Zhang and BP Murphy, Comparison of damage to human hair fibers caused by MEA- and ammonia-based hair colorants, J Cosmet Sci 65(1) 1–9 (2014)
- B Shirsath, Organic beauty market expected to rise 10%, Beauty Pack (Jan 2017)
- U.S. Pat 4,003,699, Oxidation hair dyes based upon tetraaminopyrimidine developers, D Rose, F Saygin and E Weinrich, assigned to Henkel (Jan 18, 1977)
- JF Corbett, Hair coloring processes, Cosm & Toil 106 53–66 (1991)
- C Cartwright-Jones, Addressing epidemic of p-phenylenediamine sensitization by going forward into the past, J Cosm Sci 68 48–54 (2017)
- hennaforhair.com/freebooks/hennaforhair.pdf
- https://detoxinista.com/6-things-you-
should-know-before-using-henna-hair-dye/