Small molecular weight solvents are sought in cosmetics, as it is challenging to find molecules that are efficient, safe for the skin and eyes, scarcely flammable, versatile, sensorially acceptable and compatible with most common cosmetic ingredients, all at the same time. This is why water, ethanol, glycerin and propylene glycols have been used for decades in most cosmetic formulas. Cosmetically acceptable lipophilic solvents are even rarer. In relation, one category of solvents with good potential for cosmetic applications is amphiphilic polyols. Polyols are alcohols containing multiple hydroxyl groups in their structure; these occur naturally in plants and animals, and also are produced synthetically.1
The most active industries producing new low molecular weight molecules are those working with isoprene and terpenoid structures. In nature, isoprene moieties are the building blocks for many complex molecules like terpenes and natural rubber. For industrial applications like pharmaceutical intermediates, as well as solvent blends and paints, extensive investigations on the human and environmental safety of new small size molecules have been conducted.
Of these molecules, isopentyldiol (IPD) has survived scrutiny for potential skin sensitization and irritation, eye irritation, genotoxicity and toxicity to fish, Daphnia and aquatic plants. Being subject to Japanese standards for quasi-drug ingredients,2 it also has been rated highly in terms of safety. Its safety profile together with interesting sensory properties make it a novel candidate for the personal care industry. Here, the authors investigate its properties for cosmetics and personal care applications via tactile evaluations; tests, in combination with sorbitol, to restore smoothness in damaged hair; and a skin moisturization evaluation for its capability to increase skin moisture levels.
Isopentyldiol Structure
A unique one-step synthesis technology was used to prepare isoprene from the C4 fraction of naphtha cracking, which was then put through stringent purification processes to eliminate volatile odorous fractions, yielding an original hydrophilic polyola. IPD has an isoprene diol structure. Specifically, it is a branched chain bi-alcohol with a five carbon atom backbone, described by the formula: 3-methyl-1,3-butanediol (see Figure 1). Due to its amphiphilic molecular structure, relatively small molecular size and the presence of two strategically positioned hydroxyl groups, it can form both intra-molecular and extended intermolecular hydrogen bonds, depending on the surrounding vehicle. It also has superior affinity to the skin and acts as a skin humectant and hydrotrope, as will be shown.
IPD is not a new substance; according to its supplier3 it is currently used in about 51 cosmetic products worldwide, including color cosmetics, skin and hair care and toiletries. However, it has the potential for much greater frequency—perhaps the same as for propylene glycol or similar common solvents/hydrotropes. The aim of the present study was to investigate the behavior of IPD when blended with common cosmetic ingredients and its effects on formulation properties. Evaluations were carried out to determine the material’s solvent and humectant properties, its capability to simplify manufacturing methods and its influence on the sensory performance of finished products.4
Miscibility, Sensory and Solvency
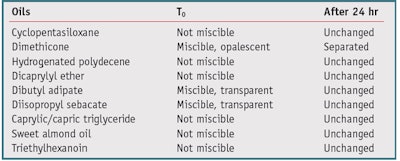
Miscibility: To test the miscibility of IPD with cosmetic oils, it was combined in a 1:1 ratio with nine fluid oil ingredients and assessed visually. The materials used were characterized by chemical structures of increasing polarity—from hydrocarbons to silicones, ethers, esters, vegetal triglycerides and fatty alcohols—and chosen for their ease of handling. The selected oils and the observed results are reported in Table 1.
Interestingly, IPD was found to be fully miscible with three of the oils tested. Indeed, it is difficult for molecules that are completely soluble in water to also be fully miscible at 1:1 ratio with large organic molecules. Further, even where miscibility was not complete, small quantities of oils could be dissolved in IPD. With the exception of ethanol, propylene glycol and glycerin do not blend with esters. IPD’s miscibility with the oils is likely due to the asymmetry of its molecular structure, having a very hydrophilic site and a completely lipophilic, non-polar site. This results in transparent phases, having no turbidity or separation.
Further, fragrances and aromas were found to be miscible with IPD at a 1:1 ratio (data not shown). In fact, the addition of 10% IPD to a hydro-alcoholic solution containing 1% fragrance reduced the amount of alcohol required to maintain transparency, from 30% to 20%. Thus, IPD could be used to create perfumes that are milder to the skin by lowering the amounts of alcohol required. It also has advantages over traditional solubilizers such as low volatility, flammability and explosion hazard, in addition to a nice sensory feel. In some countries, it even has less restrictions for use.
Sensory evaluations: A bench sensory evaluation of IPD in comparison with simple oil, ethanol and volatile silicone also was conducted. Spreading time was evaluated sensorially by applying a 0.1 mL-graduated syringe of fluid onto the inner part of one forearm. With the opposite index finger, the fluid was applied gently using circular movements, and the number of strokes was counted until resistance was first felt. Oiliness was perceived as the non-braked ease of gliding over the skin. Shine was evaluated visually by looking at the application sites under a light source.
These evaluations were only semi-quantitative, to understand the type of sensory interactions IPD had with common cosmetic oils. The scale used was from 1 (lowest value) to 5 (maximum value). Ease of spreading is expressed here as the number of strokes necessary to perceive a brake to movement (average of 5 judges, ± 10). In the case of di-isopropyl sebacate, IPD increased its ease of spreading similarly to D5, while in the case of di-butyl-adipate, it increased the ease of gliding over the skin. Shine was increased for both oils. Thus, generally, IPD increased spreading time and improved emollient feel and shine effects (see Table 2).
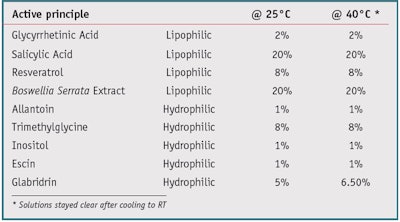
Solvent properties: Since IPD is miscible with water and some light oils, its solvent properties were tested next using hydrophilic and lipophilic actives. One gram of each active was added to 100 g of IPD and mixed to obtain a transparent solution. This addition and mixing were repeated until solutions were no longer transparent. The tests were carried out at room temperature (RT), and by heating the solution to 40°C then cooling it back to RT. The selected active principles and solubility results are expressed in % w/w in Table 3.
IPD showed good to excellent solubilizing properties of the lipophilic actives. It was able to dissolve glycyrrhetinic acid at 2%, salicylic acid at 20%, resveratrol at 8% and Boswellia extract at 20%, creating clear solutions at RT. In comparison, glycyrrhetinic acid and Boswellia are practically insoluble in water. Salicylic acid dissolves at 0.2% in water, and resveratrol dissolves at 0.3 mg/100 mL in water. IPD also efficiently solubilized the hydrophilic actives trimethylglycine (8%) and glabridin (5–6.5%). Further, maximum solubility levels generally were reached regardless of whether the solution was heated or not.
The carrier property of IPD also was evaluated as either: the maximum amount of water that could be added to the IPD/lipophilic active solutions described previously without precipitation of the actives; or the maximum amount of oil that could be added to the IPD/hydrophilic active solutions described previously, without precipitation. Results, shown in Table 4, indicate that IPD could be used as carrier for both hydrophilic actives in anhydrous systems and lipophilic actives in aqueous systems.
Wetting and Dispersion
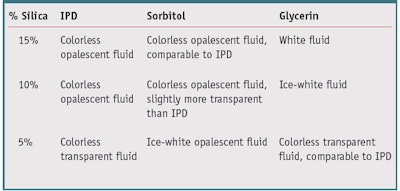
Considering that the chemical structure of IPD has polar hydroxyl groups similar to other wetting agents such as glycerol and propylene glycol, IPD was next tested in combination with pigments and fillers to determine its wetting capability. Wetting is determined by progressively adding fluid, drop by drop, to a test powder and blending it with a spatula. The trial is considered complete when the blend appears as a smooth, homogeneous paste. The selected pigments were titanium dioxideb and iron oxidec (CI 77492), and the chosen fillers were two grades of hydrated silicad and a common grade of hydrophilic fumed silicae. The dispersion ability of IPD was compared with that of glycerin and sorbitol. IPD showed good wetting of iron oxide pigments (1:1) and titanium dioxide (4:5, TiO2:IPD).
IPD provided better dispersant and wetting properties for the hydrophilic fumed silica powders than glycerin, resulting in a colorless, transparent gel. It also provided comparable dispersion of hydrated silica to sorbitol, creating colorless gels that varied from soft and transparent, to harder and opalescent. This suggests, once wetted by the solvent, an efficient swelling of the porous fillers. These results are reported in Table 5. These results suggest IPD could be suitable for use in toothpastes, both transparent and traditional, due to its good wetting properties for silica powders and its aforementioned solubilizing capacity of aromas and fragrances.
IPD in Cleansing Systems
The behavior of IPD in combination with common cosmetic surfactants also was tested (see Table 6). Evaluated parameters included foam height and stability, viscosity variations and sensory profiles. In a 400-cc beaker, 100 g of a solution at 0.5% active matter was prepared. The solution was homogenized at maximum speed for 1 min and slowly poured into a 250-cc cylinder with an internal diameter of 35 mm. The height of the foam was measured at two different times: t0 (1 min after preparation) and t1 (5 min after preparation). The stability percentage was calculated as t1/t0 x 100.
When combined with sodium laureth sulfate (SLES), IPD increased foam height and stability proportionally to its concentration. In mild surfactant systems, the best results were found with 2% IPD, which suggests IPD as a valid alternative to traditional foam boosters in surfactant systems. At 1–1.5%, IPD also had a positive influence on the viscosity values of SLES-based systems (data not shown). Further, the final skin feel of cleansing products containing IPD was less dry, with a slightly more emollient feel than reference products without the IPD.
IPD in O/W Emulsions
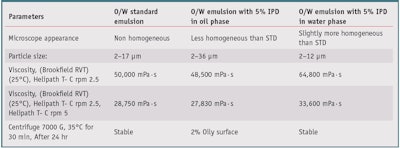
The effects of IPD on the stability of o/w emulsions were evaluated as well (see Formula 1). IPD was introduced at 5% in oil phases, in combination with the emulsifying system, or in the water phase. As reported in Table 7, when added to the water phase, IPD produced smaller-diameter oil droplets, with better homogeneity of the system, compared with the same standard emulsion without IPD, subsequently improving its stability (see Figure 2).
Skin Moisturization
The hydrating power of IPD was next examined in a preliminary clinical test of five volunteers.5 Four formulas were developed starting from a basic o/w emulsion (see Formula 2): the first omitted the moisturizing agent, the second included 5% IPD, the third included 5% IPD with 5% sorbitol, and the fourth included with 0.5% of sodium hyaluronate. A 0.1-g sample of the product was applied to the arm, and skin moisture content was measured with a corneometer after 5 min, 1 hr and 2 hr.
The formula containing 5% IPD showed an immediate, i.e., after 5 min, hydrating effect on the skin, with a 94% moisture increase. Five minutes after application, the formula combining 5% IPD with 5% sorbitol showed a 111% increase in moisturization. This synergistic association with sorbitol also extended the moisturizing effect up to 2 hr. These results showed improvement over those obtained with the traditional moisturizer sodium hyaluronate (0.5%), with a 55% increase. Results are shown in Figure 3.
Repair, Sensory in Hair
The reparative effects of IPD on damaged hair and the sensorial properties in oil-based hair cleansers6-8 also were considered. Several aqueous solutions of ingredients were tested, including: 5% IPD and 5% sorbitol; 2% hydrolyzed wheat proteins; 2% hydrolyzed silk; 5% dipropylene glycol and 5% sorbitol; 5% butylene glycol and 5% sorbitol; 5% propylene glycol and 5% sorbitol; and 5% propylene glycol and 5% xylitol. The smoothness of hair was evaluated by coefficient of static friction (sliding angle test) and drop height (ring test).
For the sliding angle test, a hair lock is fixed on a plexiglas plate and combed twice. The angle of the plate required for a 50-g weight to drop in 3 sec is determined; the higher the sliding angle, the more damage to the lock (see Figure 4). For the drop height test, a hair lock is fixed to pliers held at 30 cm high. A 6-g ring is inserted at the top of the lock. When the ring is released, the drop height of the ring is measured in cm. This test is repeated five times for each lock; the lower the drop height of the ring, the higher the damage of the hair.
Results are shown in Figure 5 and Figure 6. After treatment with 5% IPD and 5% sorbitol, the smoothness of damaged hair increased, compared with IPD alone, hydrolyzed proteins, or the addition of other glycols to sorbitol. The reparative effect obtained was also visible by microscope (see Figure 7), where IPD showed a synergistic effect with sorbitol (ratio 1:1) in the treatment of damaged hair. When combined, the two polyols improve the aspect of damaged hair fibers.
Effects in Makeup
The effects of IPD on the properties of makeup products were evaluated in preliminary tests on different types of formulations. In particular, the binding properties of powders were verified in a compact powder (see Formula 3).
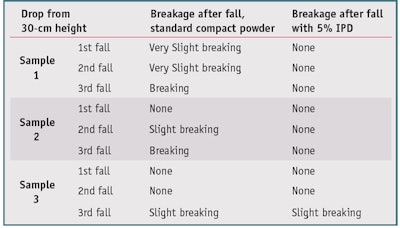
IPD was tested at 5% to verify its properties as a binder and determine its influence on sensorial performance; it was included directly in the powder blend after the addition of the binder system. Drop tests were then carried out on three samples of the formula, with and without IPD. The samples were dropped from a fixed 30-cm height, and the integrity of the samples visually assessed. As reported in Table 8, IPD significantly improved the firmness of the product and its resistance to breakage. Moreover, it improved the pick-up phase. Spreadability, trace uniformity, matte effect and after feel also remained unchanged, as assessed subjectively by five expert formulators.
Moreover, the sensorial effects of IPD in several other makeup formulations were verified, again assessed subjectively by expert formulators. In lipsticks, 2.5–3% IPD significantly improved the application performance, i.e., soft feel, slide, shine and trace without effects on drop point. However, at 5% it appeared to negatively affect product structure/stability. IPD hence supported a gliding application, uniformity of the trace and long-lasting shine effect.
In cast foundations, the addition of IPD to a difficult-to-pour foundation heated at 80-85°C (too viscous) improved the casting phase, and the skin application pick-up, spreading and sliding were also improved. Finally, in liquid foundation, IPD increased transparency and imparted a natural look without affecting spreading time, shine and massage time.
Conclusions
The aim of the present study was to investigate the behavior of IPD when blended with common cosmetic ingredients, and its effects on formulation properties. The evaluations carried out show it is easily added to many types of cosmetic formulae without inducing significant stability problems. Further, it was shown to impart many benefits, such as simplifying the addition of hydrophilic actives into anhydrous formulas and vice versa; good solvent power; the ability to decrease alcohol concentration in alcoholic perfumes; being a good foam stabilizer, silica dispersant and aroma carrier; and increasing foaming and viscosity properties in surfactant systems containing SLES. It also shows efficient skin moisturization, reparative effects on damaged hair when combined with sorbitol and improved breakage resistance. Its safety profile, together with these interesting properties, make it a novel candidate for the personal care industry.
References
- Propylene Glycol: Information Update, Propylene Glycol Panel of the American Chemistry Council, www.lyondellbasell.com/techlit/techlit/2279.pdf (July 2001) (Accessed Oct 7, 2013)
- Y Hiyama, GMP Guideline for Drugs and Quasi-drugs, National Institute of Health Sciences, www.nihs.go.jp/drug/section3/H19GMPguideline4.pdf (Accessed Oct 7, 2013)
- www.kurary.eu (Accessed Oct 7, 2013)
- Internal Report on isopentyldiol cosmetic applications, Rigano Industrial Consulting & Research, Milano (2013)
- Mise en évidence de l’action synergique du mélange isoprène glycol et sorbitol par mesure du pouvoir hydratant sur peau, 10/E2629, IRFAQ, Poitiers, France (2010)
- Mise en évidence de l’effet réparateur capillaire de compositions sur base d’isoprene glycol par mesures instrumentales, 09/E2564, IRFAQ, Poitiers, France (2009)
- Mise en évidence de l’effet réparateur capillaire du composant isoprene glycol, 09/E2587, IRFAQ, Poitiers, France (2009)
- Complément d’étude sur l’isoprene glycol—Mesures instrumentales, 10/E2626, IRFAQ, Poitiers, France (2010)