Read parts II and II of this article, adapted into one, in the September 2023 digital magazine.
Cosmetic products based on w/o emulsions are widespread and have a long history of use. For this type of emulsion, water droplets are distributed in a lipid phase. Unlike water-miscible o/w emulsions, w/o emulsions do not mix with added water without mechanical action. This has a significant impact on microbiological stability, the performance of microbiological testing and interpretation of results.
As microbiological stability is an essential aspect of product quality and plays a central role in safety assessment, questions on microbiological test methodology and corresponding result-interpretation are increasingly relevant. The present article addresses these; Part II addresses how to assess microbiological safety.
W/O Emulsions and Microbial Testing
W/O emulsions are characterized by the presence of an external, or continuous, lipophilic phase and water droplets as the internal phase—i.e., the hydrophilic, dispersed phase (see Figure 1). This emulsion type contains surface-active substances including surfactants and emulsifiers that contribute to the stabilization of the emulsion.
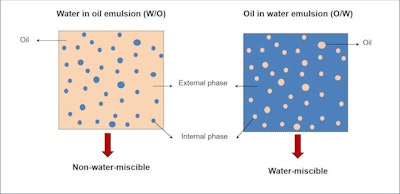 Figure 1. Schematic of w/o vs. o/w emulsions
Figure 1. Schematic of w/o vs. o/w emulsions
Microbiological testing of w/o emulsions is required for preservative efficacy tests (PETs) during product development and scaling up, as is microbial content testing during in-process controls. The PET according to the test guideline1 given in ISO 11930 is important for the microbiological product safety assessment of cosmetic products in Europe.2
The first part of the test involves the inoculation of the product with test microorganisms in an aqueous suspension. The microbial content is determined at predefined time points and reductions from the initial microbial count indicate efficacy. The determination of the microbial count in the second part of the PET consists of a microbiological content test.
However, w/o products lack water miscibility, influencing the assay results, which therefore requires special attention to the following aspects of microbiological testing to obtain reliable results:
● Preservative effectiveness test: The test microorganisms in aqueous suspension must be distributed as evenly as possible in order to ensure good reproducibility and broad efficacy of the preservative.
● Microbial content testing: In order to recover all microorganisms present, a complete "break-down" of the emulsion and sufficient mixing are necessary to ensure the even distribution of microorganisms during sampling. Also, to avoid residual antimicrobial effects, one must verify sufficient neutralization.
Limitations of Preservative Efficacy Tests in W/O Emulsions
In the case of non-water-miscible products, the first problem encountered is the adequate contamination of the product with an aqueous microbial suspension (usually 1% of the sample volume). Without any physical agitation, this suspension would simply lie on the surface of the tested product (external, lipophilic phase).
Alternative methods to achieve sufficient stirring may be considered; e.g., vortex with glass bead, homogenizer, etc. Depending on the emulsion formulation and its viscosity, different mixing outcomes may result. It is recommended to standardize stirring as much as possible, including stirring duration since intensive stirring can change or partially break down the emulsion structure, increasing the coalescence of water droplets.
While thoroughly stirring contributes to a certain uniform distribution of the test microorganisms in the product, a complete homogeneous distribution in the water phase is not possible in this type of emulsion, since the water is present in discrete droplets. On the contrary, the further formation of water droplets results (see Figure 2a), and penetration of test microorganisms into the discrete water droplets (dispersed phase) through the continuous fat phase would not be expected.3, 4
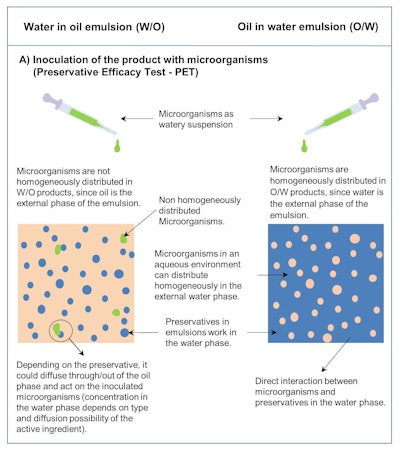 Figure 2a. Schematic of the inoculation of microorganisms as a watery suspension when performing a PET on non-water-miscible (w/o) vs. water-miscible (o/w) cosmetic products
Figure 2a. Schematic of the inoculation of microorganisms as a watery suspension when performing a PET on non-water-miscible (w/o) vs. water-miscible (o/w) cosmetic products
It is notable to consider that the inoculation step, as described for the PET, mostly represents an “artificial” test situation that fundamentally deviates from contamination under real-life conditions. During the use of w/o products and their removal by hand from packaging, significant water entry into the product remaining in the package is not expected.
During testing, on the contrary, an unavoidable amount of water is added to the product for inoculation. Moreover, mechanical stirring may impair the water droplets in the emulsion and at least partially change the protective character (limited water availability) of the emulsion. Modifications of the experimental procedure of the PET for w/o emulsions have been made, intended to approximate more true-to-life conditions.5, 6 However, systematic implementation to prove the effect of those experimental modifications is lacking.
To reduce the amount of “added” water during testing, a lower inoculation volume commonly is used (from 1.0% to 0.1%)6 although this does not change the basic conditions described above. Another consideration is to introduce contamination using a carrier (e.g., mineral oil) to avoid additional water in the emulsion system.7
To facilitate the detection of microbial proliferation or static effects, the Personal Care Products Council (PCPC) suggests reducing the initial bacterial load from 105 CFU/g to 103-104 CFU/g. However, variations in results are more likely to occur for reduced counts, rendering the microbial reduction criteria as specified in the guidelines unachievable. In this case, the reduction requirements should be set based on the original initial count.7
Collecting evidence for an antimicrobial effect based on emulsion character is nearly impossible with a PET. This is due to the added water and the test microorganisms being outside the dispersed water droplets as extrinsic contamination.
As far as a preservative effect being achieved by antimicrobial actives (i.e., preservatives, according to Annex V of the Cosmetics Regulation, other antimicrobial substances),8 the substance character of the active systems and the diffusion conditions must be considered. This is because preservation is primarily required in the aqueous phase where microorganisms can proliferate.
Most preservatives are lipophilic and they tend to accumulate in the continuous emulsion phase whereas only lower concentrations are in the dispersed water phase. Added test microorganisms are therefore not homogeneously distributed in the dispersed water droplets, and are thus unevenly exposed to effective preservative concentrations.9, 11 Consequently, areas of differing efficacy in the product may contribute to varying results when performing the tests.
Microbial Counts in W/O Formulas
Microbial counts are conducted in the product after artificial contamination (PET) at defined times to determine the death kinetics. For this purpose, at least 1 g of the inoculated test sample is taken. For the recovery of surviving microorganisms, besides mixing the product sample with the dilution medium (aqueous buffer solution), a surfactant such as polysorbate 80 or isopropyl myristate is added (ratio: 1 g product, 1 g surfactant and 8 g dilution medium) to improve water miscibility, resulting in phase inversion or emulsion break down (see Figure 2b).
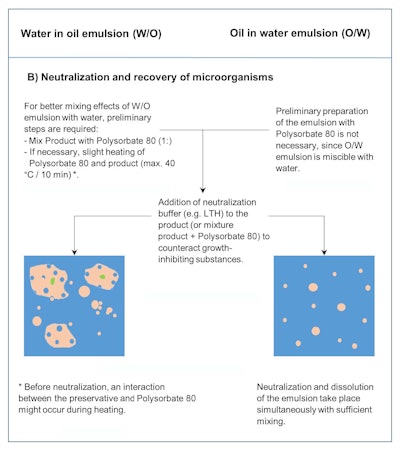 Figure 2b. Schematic of the neutralization and recovery of microorganisms when performing the PET (also used for microbial purity testing) on non-water-miscible (W/O) vs. water-miscible (O/W) cosmetic products. The second critical step of the method is the proper breaking or phase invention of the emulsion by using excess amounts of surfactant.
Figure 2b. Schematic of the neutralization and recovery of microorganisms when performing the PET (also used for microbial purity testing) on non-water-miscible (W/O) vs. water-miscible (O/W) cosmetic products. The second critical step of the method is the proper breaking or phase invention of the emulsion by using excess amounts of surfactant.
In certain cases, heating the dilution medium, surfactant and product can further increase water miscibility. However, both of these commonly used approaches require caution in their application. Heating may lead to the increased effectiveness of antimicrobial ingredients, or a high concentration of surfactant may itself exhibit antimicrobial activity or potentiate antimicrobial effects.9 Therefore, heating should always be limited in terms of temperature and time: a maximum of 40°C and 10 min. Most important with this processing step is to stop any residual antimicrobial effects of the preservative by verifying neutralization, which can be assessed using a respective validation method.1, 10
Microbial Contaminants in W/O Formulas
Microbial survival or proliferation in a cosmetic product always depends on water availability. Even though w/o emulsions are not anhydrous products, the water in the formulation is restricted to the dispersed phase, which significantly limits water availability. Thus, there is a low risk of microbial contamination because, as noted, the oil- or fat-rich base of these products, which isolates the water phase, generally does not support microbial growth.9, 11
In this context, the type and moment of product contamination are also important in terms of contamination risk. Consider there is both intrinsic and extrinsic contamination. In the case of intrinsic contamination, the introduction of contaminants occurs during the production process, i.e., before the formation of the emulsion structure. In contrast, extrinsic contamination involves the introduction of microorganisms into the finished product, i.e., into the readily built emulsion; therefore, microorganisms have limited access to water.
The literature shows that when a w/o emulsion is intrinsically contaminated, microorganisms that enter the dispersed water droplets may present a hazard to the emulsion. However, this is only possible if the microorganisms (e.g., lipases, hydrolases) can metabolize components of the emulsion and microbial growth promotes the destruction of the w/o emulsion. The release of extracellular spoilage factors (e.g., various lipids) may also contribute to emulsion break down; for example, in the case of specific Pseudomonas strains.12 It should be noted that the hazard also depends on the size and distribution of the water droplets dispersed in the oil phase: the smaller the water droplets, the more difficult it is for contained microorganisms to survive.13
Extrinsic contamination presents a different situation. Contamination occurs after emulsion formation, at a late manufacturing step or when the finished product is used. Microorganisms that enter the lipophilic external phase are usually unable to pass through this phase into the dispersed water phase, and the few water droplets with low numbers of microorganisms pose no significant hazard.14 One study confirmed this using E. coli and C. albicans as test microorganisms in a semi-solid w/o formulation.3, 4
The contamination risk assessment during product use (extrinsic contamination) is of particular importance in terms of the PET and the microbiological safety assessment (see Part II of this article, coming in August 2022). Besides this general assessment of w/o emulsions, it also is essential to consider the conditions in each individual product when evaluating microbiological product safety. This is due to wide range of formulation and manufacturing variations—e.g., type of emulsifier, stabilizers, pH dependence, phase volume ratio, droplet size distribution; and product types—e.g., lotion, high viscosity creams, etc.
Differences regarding assay as well as microbiological stability/contamination susceptibility can be assumed. Thus, for a reliable assessment of the microbiological stability of each product, it is essential to ensure a constant formulation quality and production-method (GMP requirements).
Conclusion, Part I
Although the basic microbiological stability of a w/o product can be assumed in emulsions with satisfactory quality, depending on the product characteristics, formulating with antimicrobials is, in certain cases, recommended. Consequently, despite method limitations, checking the antimicrobial effectiveness by means of PET remains required.
The limitations of the PET by w/o formulations explains, to a certain extent, the high variability repeatedly observed in test results. Part II of this series approaches this topic by comparing PET frequency failure between o/w and non-w/o formulations. It outlines how the reliable microbiological risk assessment of w/o cosmetic products requires a systematic, holistic approach without relying completely on PET results. Stay tuned for Part II of this discussion.
References
1. International Organization for Standardization (2019). Cosmetics—Microbiology—Evaluation of the antimicrobial protection of a cosmetic product (ISO 11930). Retrieved from https://www.iso.org/standard/75058.html
2. DGK (2015). Konservierung Kosmetischer Mittel–Prüfmethoden, Teststrategie und Wirkungsabsicherung. Thannhausen, Bavaria, Germany: Verlag für chemische Industrie, H. Ziolkowsky.
3. Döhling, C.H. und Daniels, R. (2012). Hygienerisiko nicht konservierte halbefester Zubereitungen zur Anwendung auf die Haut. Teil 1. Pharm Ind 74(6) 998-1004.
4. Döhling, C.H. und Daniels, R. (2012). Hygienerisiko nicht konservierte halbefester Zubereitungen zur Anwendung auf die Haut. Teil 2. Pharm Ind 74(7) 1156-1162.
5. Leimbeck, R. (1982). Mikrobiologische Qualität von Kosmetika–Aktuelle Probleme, Teil 2: Der Konservierungsbelastungstest. Parf Kosm 63 50-60.
6. Leimbeck, R. (1989) Praktische Erfahrungen mit dem Konservierungsbelastungstest. J PharmTechn 10 23-29.
7. Personal Care Products Council (2016). Microbiology guidelines. Section 20: M-6 Method for preservation testing of atypical cosmetic products.
8. European Commission (2009). Regulation of the European Parliament and of the Council on Cosmetic Products, No. 1223/2009.
9. Akers M. J. and Walcott V. K. (2007). Official methods of preservation and testing. In: Denyer, S.P und Baird R.M., eds. Guide to Microbiological Control in Pharmaceuticals and Medical Devices. Boca Raton, FL USA, CRC Press pp 384-386.
10. Eigener, U. (2021). VIII.1 Mikrobiologische qualität und produktsicherheit kosmetischer mittel. In: Baumgart, J., Becker, B. and Stephan, R., eds. Mikrobiologische Untersuchung von Lebenmitteln. Hamburg, Germany, Behr’s Verlag.
11. Yablonski, J.I. (2006). Risk factor assessment of anhydrous atypical cosmetic products. In: Orth, D.S., Kabara, J.J. and Denyer, S.P., eds. Cosmetic and Drug Microbiology. Boca Raton, FL USA, CRC Press pp 133-144.
12. O'May, G., Allison, D. and Gilbert, P. (2004). A rapid method for the evaluation of both extrinsic and intrinsic contamination and resulting spoilage of water‐in‐oil emulsions. J Appl Microbiol 96, 1124-1132.
13. Verrips, C.T. und Zaalber, J. (1980). The intrinsic microbiological stability of water in oil emulsions. Eur J Appl Microbiol Biotechnol 10 187-196.
14. English, D.J. (2006). Factors in selecting and testing preservatives in product formulations. In: Orth, D., Kabara, J. J., Denyer, S. and Tan, S.K., eds. Cosmetic and Drug Microbiology. Boca Raton, FL USA, CRC Press pp 77-109.