Glycols have been used in cosmetics and personal care products to impart beneficial properties such as humectancy, solvency, moisturization and emulsification. One such ingredient is 1,3-propanediol (PDO), which is manufactured either by a chemical process using petroleum feedstock or by a fermentation (bio-based) process using corn sugar. Since PDO became commercially available only recently, it does not yet have a widespread history of use for properties such as humectancy, moisturization or emulsification. However, a substance structurally similar to PDO, propylene glycol (PG) (see Figure 1), does have widespread use and distribution in personal care products—but it also has a history of some dermal irritation and to a lesser extent, sensitization.1, 2
Obviously, besides the efficacy properties of a raw material, another critical property is the lack of or low potential for dermal irritation and sensitization of skin. Therefore, an evaluation of the potential for new ingredients to cause adverse skin reactions is essential.3 Information from previous animal studies following exposure to chemically-produced PDO suggests a low potential for human skin reactions. This historical information includes a study in rabbits (Draize method), showing neat PDO is mildly irritating;4 and a study in guinea pigs (Landsteiner/Draize method), showing no dermal irritation or sensitization.5
To build on existing information for PDO, humans have been tested for effects to the skin using bio-based PDO of similar purity. For example, an acute dermal irritation study was conducted on 40 healthy Japanese volunteers, who received a single application of neat bio-based PDO, and no significant skin irritation was observed.6 However, acute animal and single exposure human studies are limited in their ability to predict skin reactions in humans exposed repeatedly to PDO. Therefore, human repeat-insult patch test (RIPT) dermal studies were conducted using bio-based PDO in comparison with PG to understand the potential to cause irritation and sensitization. The RIPT studies, described here, were designed using a range of concentrations and pH levels to cover a wide variety of potential personal care applications.
Materials and Methods
Test substances: PDOa (> 99.8%; CAS # 504-63-2) and PGb (USP grade purity 99.5%; CAS# 57-55-6) were obtained for the described studies. PDO was tested in two separate human RIPT involving 100 and 200 subjects, respectively. In the 200-person RIPT, PG served as a reference compound for comparison.
Modified Draize RIPT in Humans
Substance application and panel: The tested PDOa was diluted in 0.22 μm-filtered deionized water to 5%, 25% and 50% for the 100-person RIPTc; and 25%, 50% and 75% at pH 4, 7 and 9, respectively, for the 200-person RIPTd. The test solution (0.1 mL) was applied to one-inch absorbent pads and covered with a clear adhesive dressing. The strip was then pressed into place on the upper left armc or upper backd of volunteer panelists. PG at concentrations of 25%, 50%, and 75% was used for comparison with PDO in the 200-person RIPT, as noted.
Experimental design: The method for both RIPTs was similar to that described by Draize.7 For the induction phase, patches were applied to the contact sites and remained in place for 24 hr. At the end of this period, the patches were removed and the sites were examined for dermal response. An additional observation was made 24 hr later. New patches were applied and remained in place for 24 hr.
This procedure was repeated on Mondays, Wednesdays and Fridays for a total of nine applications. The same sites were used throughout the study. Following the 9th application and a 2-week rest period, a challenge application was applied in the same manner as described in the induction phase. A duplicate challenge application also was applied to a previously untreated site on the other armc or on the backd. The sites were examined for irritation and sensitization at 24 hr and 48 hr, post-removal.
Results
The 100-person RIPT study evaluated only PDO and a deionized water control. No skin reactions were observed in any study participant exposed to 5%, 25% or 50% PDO or vehicle controls during either the induction or challenge phases. Under the conditions of this study, PDO was not found to be a skin irritant, fatiguing agent or skin sensitizer.
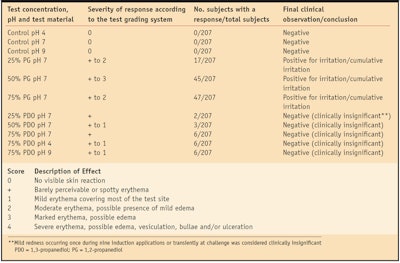
In the 200-person RIPT, no clinically significant skin reactions were observed for PDO (see Table 1). High and low pH did not impact the skin results even at 75% PDO. A few subjects exposed to 25%, 50% and 75% PDO did exhibit barely perceivable or mild redness (erythema) at 24 hr or 72 hr post-challenge, but the sites were considered clinically insignificant at the final observation. No erythema was observed throughout the induction period for 25% and 50% PDO. At 75% PDO, four individuals expressed mild erythema after one of nine applications.
Allergic contact dermatitis or sensitization was not induced by PDO or PG. However, mild, moderate and marked skin irritation and cumulative skin irritation reactions were observed throughout the testing period for all three test concentrations of PG (see Table 1). These reactions occurred during the nine applications of the induction phase and 24 and 72 hr after challenge with different degrees of severity. Some individuals exhibited a response after six of the nine applications. These results represented irritant or cumulative irritant responses in 8.2%, 21.7% and 22.7% of the test population exposed to 25%, 50% and 75% PG, respectively.
Discussion
As has been described, the potential of PDO to cause dermal effects in humans was explored in two repeat insult human studies to build upon existing animal tests and short-term human studies. Taken collectively, the data from these studies showed that PDO has low potential to irritate or sensitize human skin; additionally, its potential for these effects was lower than an incumbent glycol, PG.
A few panelists exposed to PDO in the 200-person RIPT produced barely perceptible to mild redness only once during the nine applications in the induction period, or a transient, barely perceptible to mild redness following the challenge application. The infrequent appearance of minor reactions with recovery after challenge was considered clinically insignificant by the study physician. Due to the hygroscopic nature of higher PDO concentrations, drying of the skin may explain the mild, sporadic and transient reddening observed.
The observations in the 200-person RIPT for PG were described as irritant or cumulative irritant reactions but were not characteristic of dermal sensitization. PG is a widely used ingredient that occasionally can produce contact dermatitis in a small portion of the population. While predictive animal tests did not detect potential sensitizing properties of PG for human skin, human patch tests have provided more consistent results.8 Patch test surveys aimed at evaluating patients with existing allergic contact dermatitis reported a 2–4% occurrence of sensitization to PG in the general population.9-11 The reactions observed in response to PG topical exposure in the above reported RIPT are indicative of irritation rather than sensitization, and it is not clear how many of these “irritation responses” may have represented a prior sensitization to PG from exposure to consumer products containing it.
Conclusion
PG and PDO are different molecules that have similar structures and physicochemical properties. The RIPT results described here suggest that PG may be more likely to cause skin reactions than PDO. Factors that may influence this difference in response include chemical structure and the extent and nature of exposure. It has been hypothesized12-14 that dipole moment may influence skin irritation responses, which could provide one explanation for this difference since PDO and PG have different dipole moments—the PDO molecule having greater flexibility.
The human population includes individuals with sensitive skin that are more likely to react to ingredients that are inactive for the majority of the population.15, 16 Since a larger portion of the population has already been exposed to PG through personal care products, cosmetics and food, it is possible that more consumers with sensitive skin have been exposed to PG than PDO, which could account for the relatively high incidence of contact dermatitis.
The search for cosmetic ingredients that do not produce skin reactions in humans, including sensitive subpopulations, is a continuing challenge. It is essential to introduce ingredients that have minimal potential for skin reactivity to reduce the risk of adverse skin reactions.
References
- JO Funk and MI Mariachi, Propylene glycol dermatitis: Re-evaluation of an old problem, Contact Derm 31 236-241 (1994)
- R Wolf, D Wolf, B Tuzun, Y Tuzun, Contact dermatitis of cosmetics, Clinics in Dermatology 19 502-515 (2001)
- JM Catanzaro and JG Smith, Propylene glycol dermatitis, J Am Acad Dermatol 24 90-95 (1991)
- L van Beek, Primary skin and eye irritation tests with propanediol-1,3 in albino rabbits, Centraal Instituut Voor Voedingsonderzoek for Degussa AG, no. 28412 (unpublished) (1979)
- HP Til and AMM Keizer, Sensitization potential of propanediol-1,3 and its distillation residue in guinea pigs, Centraal Instituut Voor Voeging Sonderzoek for Degussa AG, report no. R6183 (unpublished) (1979)
- K Kawai, A safety assessment of Zemea propanediol by human patch testing: A cutaneous irritation study in human volunteers, Japanese Society for Cutaneous Health (unpublished) (Jun 20, 2008)
- Draize, J. H., Appraisal of the Safety of Chemical in Foods, Drugs, and Cosmetics. In Dermal Toxicity, 46-53. Topeka, Kansas: Association of Food and Drug Officials of the Unites States (1959)
- H Lessmann, A Schnuch, J Geier and W Uter, Skin-sensitizing and irritant properties of propylene glycol, Contact Derm 53 247-259 (2005)
- JG Marks et al, North American contact dermatitis group patch-test results, 1996-1998, Arch Dermatol 136 272-273 (2000)
- JG Marks et al, North American contact dermatitis group patch-test results, 1998 to 2000, Am J Contact Dermatitis 14(2) 59-62 (2003)
- MD Pratt et al, North American contact dermatitis group patch-test results, 2001-2002 study period, Dermatitis 15(4) 176-183 (2004)
- MD Barratt, Quantitative structure-activity relationships for skin irritation and corrosivity of neutral and electrophilic organic chemicals, Toxicol in Vitro 10 247-256 (1996)
- MD Barratt, QSARS for eye irritation potential of neutral organic chemicals, Toxicol in Vitro 11 1-8 (1997)
- M Chamberlain and MD Barratt, Practical application of QSAR to in vitro toxicology illustrated by consideration of eye irritation, Toxicol in Vitro 9 543-547 (1995)
- MA Farage, A Katsarou and HI Maibach, Sensory, clinical and physiological factors in sensitive skin: A review, Contact Derm 55 1-14 (2006)
- AM Kligman, I Sadiq, Y Zhen and M Crosby, Experimental studies on the nature of sensitive skin, Skin Research and Technology 12 217-222 (2006)






!['We believe [Byome Derma] will redefine how products are tested, recommended and marketed, moving the industry away from intuition or influence, toward evidence-based personalization.' Pictured: Byome Labs Team](https://img.cosmeticsandtoiletries.com/mindful/allured/workspaces/default/uploads/2025/08/byome-labs-group-photo.AKivj2669s.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&fp-x=0.49&fp-y=0.5&fp-z=1&h=191&q=70&w=340)



