Editor's note: This is the first half of a two-part series that appeared in its entirety in the Cosmetis & Toiletries July/August 2016 issue. Read Part II here.
Overexposure to UVB (290 nm to 400 nm) and UVA (320 nm to 400 nm) can lead to damage such as sunburn, premature skin aging or even the development of cancer. Obviously, the best way to protect against these effects is to avoid UV exposure, but this is not always possible or desirable. Thus, the best adapted solutions are to wear clothes and/or use sun protection products.
Today’s consumers know the need for sun protection perfectly well. They demand multifunctional, reliable products with good UV protection, but also factors such as water-, rub-, sweat- or sand-resistance. Clearly, these factors are also assets for promoting the sales of sun care products.
In order to make such performance claims, evidence of efficacy must be provided. Typically, in vivo test methods are used, even if they are more and more criticized and often not normalized. Indeed, this is the case for the well-known sun protection factor (SPF) or UVA protection factor (UVA-PF), but these in vivo methods pose challenges related to ethics, cost and time, and there is great variability in the results from different test laboratories.
Today, a new factor of resistance is appearing in claims and demanded by consumers: wet skin application. It refers to the capability of sun care products to be applied directly to wet skin, e.g., immediately after emerging from water—with a guarantee for equal sun protection. In other words, it enables the user to skip the step of drying skin before applying sunscreen.
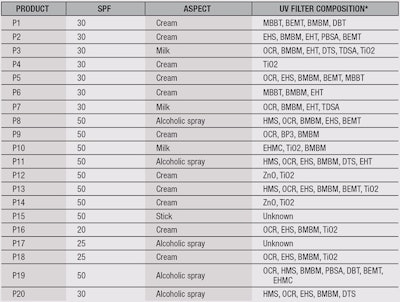
Some laboratories already perform tests in vivo to determine a Wet Skin Factor, but due reasons previously explained, a standardized in vitro method is necessary. Indeed, in vitro tests generally are more reliable because all the parameters can be controlled. The aim of this paper is to propose a reproducible and selective wet skin application in vitro test method. Here, 20 products were tested and compared according to several conditions in order to define the best parameters for the method.
Parameters of ‘Wet Skin Application’
A sunscreen claiming wet skin application must respect two important criteria: preserving solar protection and homogenous application on wet skin. These are explored in greater detail here.
Preserving solar protection: One way to consider solar protection as being preserved is when at least 50% of the sunscreen’s protection on dry skin is retained on wet skin. This relative value includes test variability strongly similar to the European Water Resistance definition.1 It assures consumers they are decently protected when they apply a wet skin product because the level of protection on dry skin can be determined using normalized methods.
Another way to claim wet skin application is based on the assimilation of the SPF measured directly on wet skin as the final protection claimed on the wet skin product. In this case, consumers must understand that when they apply a wet skin product on dry skin, the solar protection may be higher than is indicated on the product. Furthermore, this absolute value could be challenged since it depends directly of the in vivo or in vitro test method used, even if it is not normalized or adapted from a normalized method.
Homogenous application on wet skin: The second important criterion is homogenous application, preferably without leaving white marks. The latter is not a necessity because it does not put consumers in jeopardy but it is an esthetic advantage. Figure 1 shows how one sun care product leaves white marks on a wet substrate (left plate), whereas another product under the same conditions does not (right plate).
Studies have already been performed and patents issued2-5 by manufacturers regarding the development of wet skin sunscreens that do not produce white marks during application. For this goal, two criteria are important: the polarity of the components and their miscibility with water. The polarity of sunscreen components depends on both the difference of the electro negative charges and on the stereo arrangement of the molecules. The more asymmetrically the charges are distributed, the more polar the molecule.
Furthermore, the molecule’s polarity influences the physico-chemical properties; more particularly, the miscibility. Indeed, polar components are soluble amongst one another, but not with apolar components. This means sunscreens that claim wet skin application must be composed of polar components so they are miscible with water. In other words, a sun care product composed of oil, which is apolar, cannot easily provide a wet skin protection factor. Alcoholic sprays, however, and creams composed mostly of water, both of which are polar, can more easily provide wet skin protection.
Protocol Overview
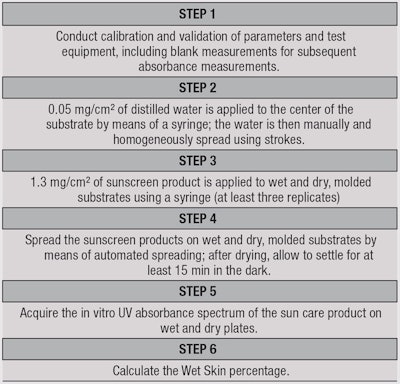
In brief, the test method developed involves measuring the SPF through a thin layer of sun care product spread onto ad hoc dry and wet substrates, and then calculating the wet skin percentage. For this, the sunscreen tested is applied directly to dry and wet polymethyl methacrylate (PMMA) platesa before robotic spreadingb. The samples are allowed to dry for at least 15 min at a controlled temperaturec, after which a spectrophotometerd is used to measure the absorbance curves of the product between 290 nm and 400 nm to determine the medium SPF.
To ensure a reliable in vitro method, several parameters were tested using different kinds of substrates, different wetting methods for plates, and different temperatures during the test and drying step. These will be described later in the article.
Calculations, Variations and Reproducibility
The in vitro SPF was calculated by means of a spectrophotometer, as described by Diffey and Robson in 1989,6 based on the combination of UV light attenuation by the sunscreen with the erythema action spectrum and a relevant solar emission spectrum. These values enable the calculation of the in vitro SPFi—the individual SPF determinate on the same substrate—according to Equation 1. In the end, the SPF of a product corresponds to the arithmetic mean of all individually validated SPFi values obtained from the tests.
Here, E(λ) is the relative effectiveness of UVR in producing erythema in human skin, according to the erythema action spectrum (CIE-1987) at wavelength λ; I(λ) is the spectral irradiance received from the UV source at wavelength λ in midday midsummer global irradiance at 40°N (W•m-2•nm-1); d(λ) is the wavelength step, equal to 1 nm in this study; and A(λ) is the monochromatic absorbance of the sunscreen layer at wavelength λ, with A(λ) = -log[T(λ)].
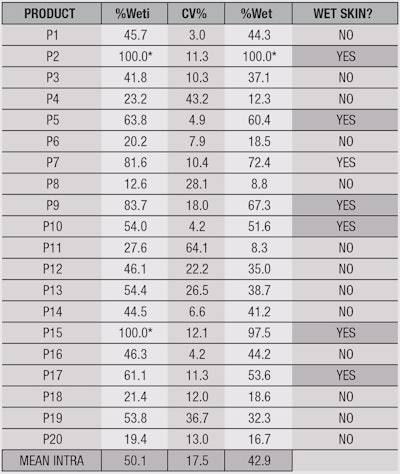
As explained previously, the method to determine the wet skin factor is based on the fact that a product is considered wet skin-applicable when it conserves at least 50% of its solar protection on wet substrate/skin, compared with its application on dry substrate/skin. For this, the initial wet skin percentage (%Weti) can be calculated according to Equation 2, which takes into account the average SPF on wet substrates (SPFwet) and the average SPF on dry substrates (SPFdry).
Nevertheless, to factor in the variability of the test, a product will be finally considered wet skin-applicable if the value for the 90% lower unilateral confidence limit of SPFwet is greater than or equal to 50% of SPFdry. In others words, by incorporating a variation factor d, it is possible to calculate the mean of the wet skin percentage (%Wet) according to Equation 3. If the value of %Wet is higher than 100%, the sun protection provided by the product on a wet substrate will be considered more efficient than on a dry substrate and, in this case, the value will remain at 100% in Table 2 with an asterisk indicating this.
The variation factor d is calculated according to Equation 4.
Here, tu is the t value from the "one-sided" student’s t distribution table at a probability level of p = 0.10 and with n-1 degrees of freedom; s is the standard deviation calculated according to Equation 5; and n is the total number of measurements in the test (equal to three replicates).
Here, SD corresponds to the standard deviation of SPFi on wet or dry substrates.
On the other hand, the coefficient of variation (CV%) will be calculated, too, according to Equation 6. This allows for the comparison of the dispersion or variation in groups of measurements.
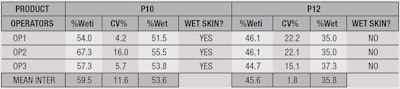
Finally, to assure the repeatability of the test, the mean of the individual CV% factors between the different products according to a same condition, called "Mean Intra," will be calculated for the development of the protocol. Furthermore, the reproducibility of this test method also was analyzed to assure that the result obtained is similar regardless of the operator or the laboratory performing the test. This was achieved by comparing the values of %Wet through the CV% or "Mean Inter," to determine the reproducibility of the test.