Editor's note: Proposed here is an in vitro method to test sunscreens for photostability. Part I sets up the protocol, Part II details the test method, calculations and results, while Part III outlines a new photostability label claim concept based on the results.
Ranking Proposal
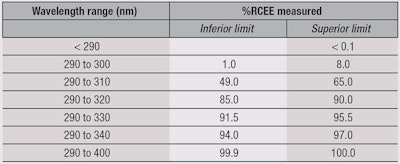
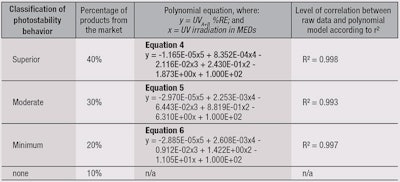
Using the results from Figure 5, photostability levels were defined and ranked in descending order based on the top-performing 40%, secondary 30%, lower 20% and bottom 10%. From this, four categories were identified (see Table 2): Superior, Moderate, Minimum and none.
The polynomial equation for each category was determined by the mean value from different products at the different UV irradiation steps for the category selected (see Table 1). As an example, the category “Superior” represented 43 products among the 107 tested (40%) showing the highest photostability behavior after classification. The polynomial equation was therefore obtained by fitting the mean value for each UV irradiation step among these 43 products.
The same process was carried out for the other categories, i.e., “Moderate” and “Minimum,” which represented 32 and 21 samples, respectively. Thus, using the previous equations in Table 2, the graph proposed in Figure 6 represents a photostability behavior ranking “abacus.”
However, as noted, determining a product’s photodegradation behavior requires more than one UV exposure to reliably rank its photostability. This is because any sun-induced photodegradation could be highly variable and dependent upon in situ circumstances, even with strict protocols. Consequently, the present method does not attempt to precisely define a product’s photostability level after one unique in situ UV exposure, but instead sets a maximum limit of photodegradation that might be considered reasonable for a product after two standardized exposures—4 MEDs and 8 MEDs.
In theory, any two different irradiation doses could be selected; however, the authors chose these as an interesting compromise based on both a practical amount of time in the laboratory, and minimum requirement to ensure reproducibility. The first exposure at 4 MEDs also was retained from the 2011 U.S. Food and Drug Administration (FDA) Monograph, which specified that a fixed pre-irradiation dose equivalent to 4 MEDs led to complete degradation of UV filters.17
Control Comparison
To check that the present method is determining only photodegradation behavior and excluding drying influences, e.g., film-forming modifications, natural deterioration of the formula or filter, etc., the least photostable product was tested with and without UV irradiation exposure; i.e., under the same conditions but with only one sample exposed to the UV lamp. The results, shown in Figure 7, confirm that without UV irradiation, the product maintained the same level of photoprotection, with a UVA+B %RE equal to 100% for 4 MEDs or 8 MEDs. In comparison, the UVA+B %RE of the product exposed to UV irradiation was just 23%. To conclude, it seems that even in the worse case, the present method successfully determined only the photodegradation behavior for the two UV irradiation doses selected.
Labeling Proposal
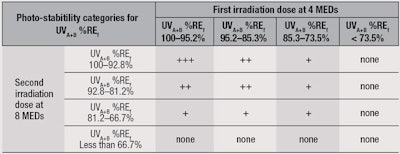
Table 3 therefore summarizes the minimum requirement of UVA+B %REf for the two irradiation steps using the proposed test method and a possible photostability ranking based on a number of plus symbols (+). Table 4 offers a logo depiction for product labels based on the results from Table 3. Through this proposal, manufacturers could display photostability information for consumers in a simple manner to indicate the reliability of the sun protection provided during use.
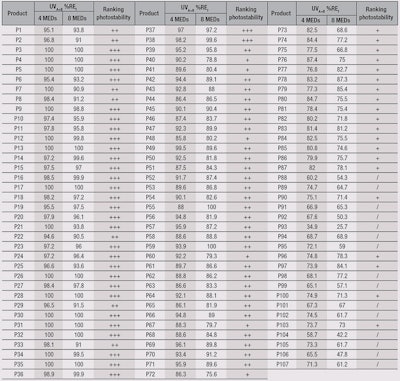
Using the present method, the UVA+B %RE for all products at 4 MEDs and 8 MEDs, and photostability ranking are shown in Table 5. Note that in order to allocate plus (+) ratings, both the first and second exposure UVA+B %RE must be considered. For example, product P60 had a UVA+B %RE of 92.2% at 4 MEDs but 79.3% at 8 MEDs, leading to the final rating of only “+”.
It also should be noted that while the most obvious effect of progressively exposing a sunscreen to UV is a reduction in its sun protection properties, in some cases, an increase in protection might be observed. Indeed, during decomposition or photo-transformations, UV filter molecules directly or indirectly create new compounds—desired or not—that can impart such effects.
Coefficient of Variation
Considering reproducibility, which is a prior condition for any proposed photostability test method, the variability of the UVA+B %RE results were compared using coefficient of variation percentages (CV%) for the 4 MED and 8 MED irradiation steps. An overview of the CV% for each type of substrate and product is shown using box and whisker plots in Figure 8. Such univariate representations provide a simple and complete representation of the minimum, first quarter, median, mean and third quarter results, displayed together with limits beyond which values are considered anomalous. The mean is displayed with a plus (+), and a black rectangle in the box corresponds to the median.
According to these results for UVA+B %RE values, the mean variations expressed by CV% between different plates were very low; approximately 0.2% and 0.3% for 4 MEDs and 8 MEDs, respectively. The results also confirmed this variability is product-dependent; although regardless of the product, the maximum variation rose to 1.8% and 2.3% for 4 MEDs and 8 MEDs, respectively. Note that these results correspond to the lowest photo-stable products and are considered anomalous from a statistics point of view. Thus, by mastering the key parameters described, this method is highly reproducible.
Conclusion
Proposed here is a test method to determine the photostability behavior of sunscreens based on a minimum of two UV irradiation doses: 4 MEDs and 8 MEDS. For 107 commercial sunscreen products, this behavior was examined and the results were used to rank the products based on the residual efficiency of their UV A+B parts, or UVA+B %RE.
By tightly controlling different key parameters, the method provided consistent and reproducible results; a very low UVA+B %RE CV% was observed, with only an average of 0.2% and 0.3% for 4 MEDs and 8 MEDs, respectively. Obviously, it is a priority to obtain absolute results; however, if the different key parameters are not strictly followed, the reliability of the method could be challenged.
By studying such a large number of products, three different photodegradation behaviors were observed: linear and polynomial photodegradation, as well as no degradation. In addition, the degradation differently impacted UVB alone, UVA alone, or both UV A+B. Fortunately, the majority of sun care products tested showed good photostability results. These results were then used to propose a pragmatic way, on finished product labels, to communicate photostability to consumers: using a photostability “plus” (+) rating as a new product claim.
Finally, this study confirmed the need to control the photostability behavior of sunscreen products for the safety of consumers, which in turn, should save on costs and improve the efficacy of ad hoc UV filter combinations used during sunscreen product development.
~Cosmetics & Toiletries
References
17. US FDA, Sunscreen drug products for over-the-counter human use, Final Rule 21 CFR parts 201 and 310, Federal Register 76 (117) (Jun 17, 2011)