A quiet scientific revolution has been taking place over the last few years as a consequence of strides in genomic science and molecular techniques. These advances have transformed the way humans view themselves as biological organisms. This revolution is the discovery of the human microbiome and with it, the realization that the human body is not simply composed of innate cells and the matrices they produce; i.e., collagen, bone minerals, etc. The body is also composed of a plethora of microbes, in numbers many times that of one’s “own” cells. This idea has been addressed by the popular press, and appears to be reaching the general public now.
Most of what is known about what is collectively called the human microbiome—that is, the ensemble of all microbes and their genetic elements, living interdependently with human cells in the host—was learned by studying the gut. However, other epithelial tissues such as the oral cavity, vagina and skin are known to be areas harboring large numbers of microbes. What is particularly interesting about the skin is that its diverse properties across different sites provide different microenvironments for several types of microbial ecosystems.
For example, the composition of the microbial population in a dry skin area, such as on the leg or arm, is different than that of a moist skin area (such as the axillae), or that of an oily and sebaceous area, such as the face or the chest.1 Moreover, over time, there is a larger interpersonal variability in the composition of skin microbial populations than there is intrapersonally.2, 3 This is one more piece of evidence indicating the microbiome is not only a “passenger” on the host organism but an active contributor to an organism’s phenotype. The idea that humans are “superorganisms” composed of taxonomically diverse ecosystems argues against the simplistic view of “good” and “bad” microbes. Thus, researchers are now looking more at the microbiome as a whole rather than separating out individual species.
Many organisms previously thought to be pathogens, such as Staphylococcus aureus, have been identified as members of microbial populations isolated from healthy skin.4 On the other hand, some species believed to be “commensals” are known to become opportunistic pathogens given the right environmental stimuli; for example, Staphylococcus epidermidis can form biofilms on catheters during infant intubations.5
New research hints that a diseased state may be achieved by the absence of commensal bacteria and not simply the presence of a pathogen. Researchers are therefore examining microbial diversity to assess health and disease.6 The emergence of the new field of skin microbiome research is beginning to define a way to describe skin health and disease. How this applies to the study of infant skin and what it means for the development of suitable infant skin care products is the subject of the following discussion.
Skin Microbiome and Infant Health
Infant skin is often portrayed as the ideal condition of beautiful skin. This may be the case but at the same time, infant skin is known to be prone to inflammatory conditions that are less frequent or absent in adults, such as impetigo, atopic dermatitis and diaper dermatitis, to name a few. The involvement of microbial infections in such conditions has been documented since the beginning of the 20th century. Recommendations for optimal neonatal care such as caregiver hygiene—particularly hand-washing before handling infants, regular bathing and umbilical cord stump disinfection—have reduced the risk of skin infections in newborns in the developed world.7 However, the incidence of infantile atopic dermatitis has risen,8 possibly due to excessive cleansing, as the “hygiene hypothesis” postulates.
This latter idea may be linked to the recent realization that microbial communities may contribute to skin homeostasis by directly modulating inflammatory responses.9 The importance of early bacterial skin colonization on infant health is becoming understood through experiments in which the health history of infants born vaginally is compared with those born by Cesarean section; birth by Cesarean section may be associated with asthma and atopic sensitization in childhood.10, 11 Moreover, it appears that the mode of delivery strongly affects the composition of the microbial ecology of newborns within 24 hr after birth, with the microbiome of infants delivered vaginally resembling that of their mother’s vaginal canal, and the microbiome of infants delivered by Cesarean section relating more to their mother’s skin.12 Taken together, such observations may underscore the importance of skin bacterial communities in modulating cutaneous homeostasis by critically affecting the maturation process of immunological reactions.
Skin Maturation and Microbial Diversity
A previous article described the changes infant skin undergoes during the first years following birth;13 the dramatic change occurring immediately after birth, from the aqueous environment of the womb to the dry atmospheric environment, requires rapid accommodation. However, the maturation process of skin continues through the first years of life, as its fundamental structure,14 biochemical composition15, 16 and function14, 15 adapt more mature, adult-like properties. Most importantly, the skin’s barrier function to external penetration, as expressed by the water-handling properties of the stratum corneum (SC), appears to be weaker than an adult’s at first, and progressively matures over the first years of life.15
More recently, the authors reported that the infant skin microbiome is also undergoing dynamic changes during the same period, with microbial populations becoming increasingly diverse with age.17 Metagenomic analysis of the infant skin microbiome confirmed what was known by traditional culture methods18—that early colonization is dominated by Staphylococcus and Streptococcus species. However, recent data indicates that the significant decline of staphylococcal species as the infant ages results in increased microbial population evenness by the end of the first year.17
In contrast to adult skin dominated by Actinobacteria,2 infant skin is colonized primarily by Firmicutes.17 Perhaps because infant skin is, in general, more hydrated and less sebum-rich than adult skin, its microbiome resembles more moist adult skin sites such as the antecubital fossa (inside the elbow) and the popliteal fossa (behind the knee).1 These early colonizers may take advantage of their preferred skin environment and then play a pivotal role in denying access to potentially infectious microbes.17
As expected, there are skin site differences in the infant skin microbiome community composition just as there are in adults. The microbiome of the diapered area includes microbes commonly found in the gastrointestinal track, e.g., Enterococcus, Clostridium and Faecalibacterium, whereas the microbial composition of the arm and the face of infants are dominated by Staphylococcus and Streptococcus.17
Microbiota Interactions with Skin
There are several symbiotic ways that microbes residing on skin may interact with cells. One is to provide developing infant skin the epitopes to train the adaptive immune system19 as it matures during the early years of life.20 Skin microflora can produce bioactive substances that have a direct effect on skin barrier function. For example, it is known that certain bacteria classes produce lactic acid, a multipotent molecule commonly found in the SC that also is considered a component of skin’s natural moisturizing factor.21
Lactic acid is a humectant, a pH buffer and, being a simple alpha hydroxy acid, a stimulator of keratinocyte proliferation.22, 23 Its L-isomer has been reported to stimulate ceramide biosynthesis, resulting in a superior skin barrier.24 Interestingly, the lactic acid bacterium Streptococcus thermophilus also has been shown to enhance levels of ceramides in keratinocyte cultures and in vivo.25 Some microbial species may also be involved in and contribute to the lipid metabolism that takes place in the SC through the production of lipolytic enzymes.26 Moreover, proteases secreted by certain species can also affect skin barrier function27 by breaking down SC proteins, including corneodesmosomes.
Bacteria can exist either in a planktonic state, i.e., as free-floating single cells, or as organized and diverse communities forming a biofilm. Recent studies report the existence of biofilms on healthy skin as well as in a number of dermatological conditions.28 Bacteria communicate with each other within biofilms through the exchange of small molecules, a property referred to as quorum sensing; however, there remain many unanswered questions about how these molecules may affect the function of human epidermal cells.
Reciprocating the action, human skin cells can also affect skin microbiota. First and foremost, the formation of a barrier to entry is of high importance. A recent study found that when common skin bacteria were added to epidermal equivalents, the virulence of bacteria depended on their access to viable layers. Specifically, when commensal bacteria were placed on the top of the SC, their effect on the release of inflammatory molecules and their cytotoxicity were minimal. However, when the bacteria were added to the culture medium, simulating the condition of a barrier breech, their cytotoxicity was dramatically increased.29, 30
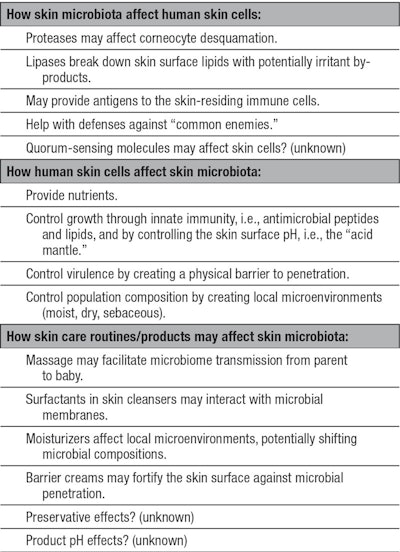
Another important way the human epidermis actively checks the uncontrolled proliferation of microbes on the skin surface is through the production of antimicrobial peptides and antimicrobial lipids.31, 32 It is important to note that one of the proposed roles of Vernix caseosa is to control early colonization through antimicrobial molecules.33 Also, Walker et al. reported that the concentration of lysozyme, a known host-defense protein and part of the innate immune system of the skin, is five times higher in neonatal skin compared with an adult’s.34 A summary of potential interactions between human skin cells and microbiota is shown in Table 1.
Implications for Baby Skin Care
The more that is learned about the early skin microbiome, the more it is being realized to be an integral part of human skin, playing an active role in skin homeostasis. As discussed previously, the infant skin microbiome may affect the healthy development of skin as it matures over the first years of life. As a consequence, the effects of infant skin care on the development of the infant skin microbiome must be addressed.
It has long been hypothesized that microbial transmission happens from caregivers to infants.7, 18 Recently, the authors also reported that skin bacteria are common between family members, which may be another way to inherit one’s genotype.35 In this work, it was demonstrated that the same strains of Propionibacterium acnes, a common skin commensal, are present in mothers and infant pairs and, in some cases, were also detected in fathers. Another recent study confirmed the transmission of the predominant skin yeast species Malassezia from mother to neonate.36 Skin microbial transmission may happen through everyday skin contact, but also may be enhanced during skin care routines such as massage.
The case for adequate preservation in skin care products is all the more important for infant care due to the immaturity of both their immune system and skin barrier. In one case study, a two-year-old patient with atopic dermatitis who had multiple abscesses, impetiginized eczematous lesions and Staphylococcal septicemia did not respond to intravenous antibiotics. The investigators were able to track the infection to the use of an unpreserved cream that was contaminated with Staphylococcus aureus. When use of the contaminated cream was discontinued, the patient’s lesions rapidly disappeared.37 The need for preservation is known in the industry but how different preservative systems may affect the skin microbiome is an entirely new area of research.
It also is conceivable that other components of skin care products may affect the skin microbiome. For example, surfactants, particularly charged polymers, may interfere with microbial membranes that are also charged.38 Moreover, harsh cleansers can damage the skin barrier, potentially offering access for skin surface microbes to the viable epidermis, where they can express their virulence.29 On the other hand, barrier-strengthening ingredients could support the skin defense system against infections, keeping opportunistic invaders in a niche where they are not a potential danger to the host and may provide benefits. Humectants may affect the composition of skin microbial communities by changing the microenvironment of the skin site upon application. Thus, notions of mild and gentle care for infant skin can now be applied to the design of products in a manner that does not disturb the normal skin development, including the microbiome.
Finally, in areas around the world where infant skin infections are still a frequent problem, anti-infective properties would be desirable for skin care products. In these cases, it is still important to note that skin microbiome diversity and richness must remain in a healthy state—the third part of Table 1 summarizes the potential effects of common skin care routines and products on the skin microbiome.
Summary
Advances in metagenomics have provided the tools to uncover a new understanding of human physiology: the notion of a “superorganism” comprising both human and microbial components. Science is just beginning to explore the microbial component in infant skin development, in which the observed evolution of the skin microbial ecology may have significant effects on the development of a healthy immune system. Microbial transmission from caregivers to infants during regular skin care routines may affect the development of the individual skin microbiome. Therefore, the design of infant skin care products should take into account how the formulation may affect the community structure of the healthy skin microbiome.
References
- EA Grice et al, Topographical and temporal diversity of the human skin microbiome, Science 324(5931) 1190-1192 (2009) .
- Z Gao, CH Tseng, Z Pei and MJ Blaser, Molecular analysis of human forearm superficial skin bacterial biota, Proc Natl Acad Sci USA 104(8) 2927-2932 (2007)
- EK Costello, CL Lauber, M Hamady, N Fierer, JI Gordon and R Knight, Bacterial community variation in human body habitats across space and time, Science 326(5960) 1694-1697 (2009)
- AL Cogen, V Nizet and RL Gallo, Skin microbiota: A source of disease or defense? Br J Dermatol 158(3) 442-455 (2008)
- GY Cheung and M Otto, Understanding the significance of Staphylococcus epidermidis bacteremia in babies and children, Curr Opin Infect Dis 23(3) 208-216 (2010)
- M Rosenthal, D Goldberg, A Aiello, E Larson and B Foxman, Skin microbiota: Microbial community structure and its potential association with health and disease, Infect Genet Evol 11(5) 839-848 (2011)
- R Sidbury and GL Darmstadt, Microbiology, in Neonatal Skin, SB Hoath and H Maibach, eds, Marcel Dekker, New York, NY (2003) pp 21-46
- AJ Mancini, K Kaulback and SL Chamlin, The socioeconomic impact of atopic dermatitis in the United States: A systematic review, Pediatr Dermatol 25(1) 1-6 (2008)
- Y Lai et al, Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury, Nat Med 15(12) 1377-1382 (2009)
- O Kolokotroni, N Middleton, M Gavatha, D Lamnisos, KN Priftis and PK Yiallouros, Asthma and atopy in children born by Caesarean section: Effect modification by family history of allergies—A population based cross-sectional study, BMC Pediatr 12 179 (2012)
- CS Benn et al, Maternal vaginal microflora during pregnancy and the risk of asthma hospitalization and use of antiasthma medication in early childhood, J Allergy Clin Immunol 110(1) 72-77 (2002)
- MG Dominguez-Bello et al, Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns, Proc Natl Acad Sci USA 107(26) 11971-11975 (2010)
- GN Stamatas and K Martin, Baby skin vs. adult skin structure, function and composition, Cosm & Toil 124(4) 50-53 (2009)
- GN Stamatas, J Nikolovski, MA Luedtke, N Kollias and BC Wiegand, Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level, Pediatr Dermatol 27(2) 125-131 (2010)
- J Nikolovski, GN Stamatas, N Kollias and BC Wiegand, Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life, J Invest Dermatol 128(7) 1728-1736 (2008)
- MC Mack, NK Tierney, E Ruvolo, Jr, GN Stamatas, KM Martin and N Kollias, Development of solar UVR-related pigmentation begins as early as the first summer of life, J Invest Dermatol 130(9) 2335-2338 (2010)
- KA Capone, SE Dowd, GN Stamatas and J Nikolovski, Diversity of the human skin microbiome early in life, J Invest Dermatol 131(10) 2026-2032 (2011)
- JJ Layden, Bacteriology of infant skin, in Neonatal Skin: Structure and Function, H Maibach and E Boisits, eds, Marcel Dekker, New York (1982) pp 167-181
- G Marchini, A Nelson, J Edner, S Lonne-Rahm, Stavréus-Evers A and K Hultenby, Erythema toxicum neonatorum is an innate immune response to commensal microbes penetrated into the skin of the newborn infant, Pediatr Res 58(3) 613-616 (2005)
- PG Holt and CA Jones, The development of the immune system during pregnancy and early life, Allergy 55(8) 688-697 (2000)
- AV Rawlings and CR Harding, Moisturization and skin barrier function, Dermatol Ther 17 Suppl 1 43-48 (2004)
- N Nakagawa, S Sakai, M Matsumoto, K Yamada, M Nagano, T Yuki, Y Sumida and H Uchiwa, Relationship between NMF (lactate and potassium) content and the physical properties of the stratum corneum in healthy subjects, J Invest Dermatol 122(3) 755-763 (2004)
- WP Smith, Epidermal and dermal effects of topical lactic acid, J Am Acad Dermatol 35(3 Pt 1) 388-391 (1996)
- AV Rawlings et al, Effect of lactic acid isomers on keratinocyte ceramide synthesis, stratum corneum lipid levels and stratum corneum barrier function, Arch Dermatol Res 288(7) 383-390 (1996)
- L Di Marzio, B Cinque, C De Simone and MG Cifone, Effect of the lactic acid bacterium Streptococcus thermophilus on ceramide levels in human keratinocytes in vitro and stratum corneum in vivo, J Invest Dermatol 113(1) 98-106 (1999)
- KE Jaeger, S Ransac, BW Dijkstra, C Colson, M Van Heuvel and O Misset, Bacterial lipases, FEMS Microbiol Rev 15(1) 29-63 (1994)
- Y Hirasawa et al, Staphylococcus aureus extracellular protease causes epidermal barrier dysfunction, J Invest Dermatol 130(2) 614-617 (2010)
- N Vlassova, A Han, JM Zenilman, G James and GS Lazarus, New horizons for cutaneous microbiology: The role of biofilms in dermatological disease, Br J Dermatol 165(4) 751-759 (2011)
- P Duckney, HK Wong, J Serrano, D Yaradou, T Oddos and GN Stamatas, The role of the skin barrier in modulating the effects of common skin microbial species on the inflammation, differentiation and proliferation status of epidermal keratinocytes, BMC Research Notes 474 6 (2013)
- Ibid Ref 29
- RL Gallo and KM Huttner, Antimicrobial peptides: An emerging concept in cutaneous biology, J Invest Dermatol 111(5) 739-743 (1998)
- DR Drake, KA Brogden, DV Dawson and PW Wertz, Thematic review series: Skin lipids, Antimicrobial lipids at the skin surface, J Lipid Res 49(1) 4-11 (2008)
- HT Akinbi, V Narendran, AK Pass, P Markart and SB Hoath, Host defense proteins in Vernix caseosa and amniotic fluid, Am J Obstet Gynecol 191(6) 2090-2096 (2004)
- VP Walker, HT Akinbi, J Meinzen-Derr, V Narendran, M Visscher and SB Hoath, Host defense proteins on the surface of neonatal skin: Implications for innate immunity, J Pediatr 152(6) 777-781 (2008)
- K Capone, M Green, GN Stamatas and J Nikolovski, Vertical transmission of skin microflora during the first year of life (poster), presented at the 21st Congress of the European Academy of Dermatology and Venereology, Prague, Czech Republic (Sep 27-30, 2012)
- R Nagata, H Nagano, D Ogishima, Y Nakamura, M Hiruma and T Sugita, Transmission of the major skin microbiota, Malassezia, from mother to neonate, Pediatr Int 54(3) 350-355 (2012)
- A Sultan et al, A case of life-threatening infections due to preservative absence in a topical cream and audit demonstrating magnitude of the problem, presented at the 26th International Pediatric Association Congress of Pediatrics, Johannesburg, South Africa, Pediatr News (suppl) 18 (2010)
- D Hoffman, M Chen, A Smiltneek, M Foegen and D Koenig, Infant skin microbiology and its relation to skin health, Cosm & Toil 123(12) 45-52 (2008)