At a first glance, teeth impact appearance. Healthy teeth support self-confidence, well-being and beauty and in most cultural groups, white, shiny and healthy-looking teeth make a positive impression. The visible crowns of teeth are covered by an up to 3-mm thick enamel layer comprising approximately 96% of the mineral hydroxyapatite. Although hydroxyapatite is the hardest substance in the human body, teeth are exposed to continuous aging by physicochemically driven, constant wear of the enamel.
Enamel is built up early during ontogenesis and it cannot regrow. Removed enamel is substantially lost and this loss may become a problem over the years; aged teeth begin to look dull and sensitivity can develop. The two primary triggers responsible for dental wear are: an acidic environment and mechanical forces.
Hydroxyapatite: Due to its chemical structure, hydroxyapatite is sensitive to acids, which destroy it by releasing calcium ions and hydrogen phosphates. Hydroxyapatite demineralizes at pH values below 5.5.1 Such demineralization occurs either by exposure to bacterial metabolites, leading to cavity formation, or by consuming acidic nutrients, which induces enamel erosion.2 Further, while hydroxyapatite is harder than other minerals, rating a moderate 5 on the Mohs scale,3 substances of equal or greater hardness can mechanically remove enamel by abrasion.
Repair technologies: To meet consumers’ needs for products repairing damaged or refilling worn enamel, several technologies were marketed in toothpastes. Fluoride is the most important one and its main benefit is remineralization. Following the demineralization of hydroxyapatite, due to a low pH, fluoride interacts with the remaining apatite and forms fluoridated hydroxyapatite. Fluoridated hydroxyapatite improves enamel hardness and resistance to acidic attacks, in turn reducing the risk of cavity formation.4, 5
To repair small fissures or refill worn enamel, zinc hydroxyapatite is another technology introduced, which due to its biomimetic structure, is reported to bind to the surface of enamel and close small microscopic defects.6 The toothpaste containing it does not contain fluoride, most likely because the hydroxyapatite would react with the fluoride ion and form fluorapatite within the formula.
The combination of both approaches in one formula—i.e., repairing demineralized and refilling worn enamel—would be a significant improvement for oral care. As such, presented here is a fluoride-compatible technology, shown to repair damage and refill micro lesions in tooth enamel. This liquid enamel technology is composed of calcium glycerophosphate and a unique carboxymethyl cellulose.
To develop this technology, several types of carboxymethyl cellulose were screened for their ability to build film on the enamel surface. As a result, a carboxymethyl cellulose with a low viscosity and a degree of substitution between 0.5 and 1.0 was determined the best option for the desired application. To investigate its enamel-repairing effect, the authors performed qualitative analyses using scanning and transmission electron microscopy, as well as quantitative analyses measuring enamel hardness. In addition, the refilling of micro lesions in enamel was assessed by profilometry.
Material and Methods
Test formulas: The placebo and test formulations for the described studies consisted of water, hydrated silica, humectant, surfactant, buffer, thickening agent, aroma and 1,450 ppm fluoride. The test formula also contained calcium glycerophosphate and the carboxymethyl cellulose.
Microscopy sample preparation: Slices of enamel were generated from retained molars of human teeth, which were embedded in epoxy resin and subsequently polished with silicon carbide to obtain planar enamel surfaces. These surfaces were initially demineralized by exposure to 1% citric acid (pH 3.8) for 15 min based on the method of Ganss et al.7 This procedure replicates superficial “early erosion” demineralization, simulating conditions that can occur in oral milieu every day.
Following this initial demineralization, enamel samples were cyclically treated with the test or placebo formula for five days with six cycles per day. Between treatments, samples were stored in a buffer solution according to Chung et al.8
Nano-indentation measurement: Using a nanoindentera, the micro hardness and indentation modulus of the treated enamel surfaces were quantified in continuous stiffness mode to a maximum depth of 3,000 nm.
Scanning and transmission electron microscopy: To qualitatively assess the reparative effects of the formulas on enamel surfaces and in cross-sections, scanning electron microscopy (SEM)b and transmission electron microscopy (TEM)c were utilized. The latter was performed on an ultrathin (approx. 100 nm) cross-section layer prepared by a focused ion beam (FIB); for this technique, the enamel surface was coated with platinum before a focused beam of gallium ions (Ga+) successively cut the layer at a profile depth of 5-10 μm.
Surface profilometry sample preparation: Four bovine enamel specimens were embedded in an acrylic resin and polished to ensure a standardized surface. For introducing micro lesions, a defined enamel area was etched by citric acid (5%) for 30 sec while the neighboring enamel was protected by adhesive taping.
Profile measurement: To measure baseline values, the tape and residual adhesive was thoroughly removed and the surface profile was scannedd instrumentally, 180 times at intervals of 0.025 mm over the whole specimen. For assessing baseline, the average height difference between intact and etched enamel was calculated.
Next, the toothpastes were applied to the specimen while protecting the intact enamel by taping, as before. Samples were brushed for 1 min with a toothpaste:water 1:1 slurry and a load of 150 g. This procedure was repeated twice daily for a total of 2.5 days while the samples were stored in artificial saliva at 37°C during the brushing periods. Measurements were repeated and height differences were assessed as described before. For assessing the longevity of product performance, the same specimens were exposed to ultrasound for 5 min and measured as before.
Results: Repair Effect
The resistance of enamel against physicochemical forces can be assessed by measuring two endpoints: hardness and indentation modulus. Nano-indentation showed a more pronounced hardness of enamel surfaces treated with the test formula, compared with the placebo (see Figure 1). The effects of the test formula on the indentation modulus were even more pronounced—the treatment positively affected the demineralized enamel as the surface became harder and the indentation modulus increased.
To assess these physiochemical effects qualitatively, images of the various surfaces were taken using SEM (see Figure 2). In Figure 2a, the smooth surface of intact enamel is shown, which turns into a roughened and porous-appearing surface after the acid challenge (see Figure 2b). Here, the boundaries of hydroxyapatite prisms becoming visible due to the acid. After placebo treatment (see Figure 2c), the surface appears similar but more diffuse, irregular and rough.
Following treatment with the test formula, however, the demineralized enamel surface (Figure 2d) appears smooth and homogenous due to particles on the surface. The ultrathin cross-section allowed for a detailed investigation of microstructure volume near the surface as function of profile depth in TEM (see Figure 3).
These results support the previous findings; while the demineralized enamel showed a porous surface structure compared with intact enamel, the test sample-treated enamel had an overall more compact structure on the surface, which was observed up to 100 nm in profile depth (see Figure 3).
Results: Filling Effect
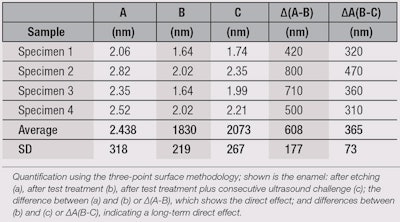
After testing for repair efficacy, the authors assessed whether the test product could refill micro lesions within enamel. Figure 4 shows profilometric scans of the: a) baseline, with an etched area in the middle and neighboring intact enamel; b) test sample-treated specimen; and c) test sample-treated specimen subjected to ultrasound. Differences in the profile heights are visualized by colors, where blue indicates a high level and red a low level. Calculating the height differences further supports this visual impression (see Table 1).
Shown are the profile heights of specimens a, b and c, along with the calculated differences between a and b (∆a-b), which reveal an immediate effect; and between b and c (∆b-c), indicating a long-term effect. Following treatment of the etched enamel with the test formula, the micro lesions were refilled in each of the investigated specimens, with an average layer of up to 608 nm (± 177 nm). Further, this layer remained even after a 5-min ultrasound challenge, with up to 365 nm (± 73 nm) thickness.
Discussion
Only when both enamel repair and refilling occur can substantial tooth damage be reversed.9 Thus, in this study, the authors assessed the ability of a toothpaste incorporating a unique ingredient—i.e., a combination of calcium glycerophosphate with a carboxymethyl cellulose—to both repair demineralized and refill worn enamel.
Since the mechanical behavior of enamel is a function its hardness and indentation modulus, nano-indentation was used to observe improvements in tooth hardness, while the indentation characteristics of enamel surfaces treated with the test sample were compared with a placebo (see Figure 1).
Quantitative analyses were further supported by qualitative analytical methods; these included the visualization of enamel surfaces by SEM (see Figure 2) and cross-sections by TEM (see Figure 3). Such techniques revealed a a more dense surface modification to the enamel structure after previously demineralized enamel was treated with the test formula.
The refilling of micro lesions in damaged enamel was further shown by profilometry. Here, micro lesions were treated with the test formula and found, through ultrasound, to provide a lasting enamel-refilling effect. Based on these placebo-controlled, in vitro results, it seems the proprietary liquid enamel technology evokes beneficial effects on demineralized and etched enamel.
Most likely, the negatively charged carboxymethyl cellulose binds to the predominantly positively charged hydration layer of the enamel surface,10 building up a thin layer. Since carboxymethyl cellulose also has ion-exchanging abilities, it could be binding ions from the toothpaste such as fluoride or calcium cations, storing a reservoir next to the enamel; this supports remineralization as it becomes necessary.
To investigate this mechanism in depth, electron dispersion x-ray (EDX) was used to analyze the modified surface layer of the test sample-treated specimen. This technique further supported the hypothesis: a relative fluoride enrichment, compared with demineralized or intact enamel surfaces, was shown. This initial analysis provides additional evidence of the liquid enamel technology helping to improve enamel repair processes. And due to its refilling, layer-building effect, the technology is of interest for additional oral care benefits, such as sealing open dentine tubules and therefore reducing tooth sensitivity.
Conclusion
In conclusion, the results show that a fluoridated toothpaste containing the specialized liquid enamel technology helps to repair damaged enamel and further refill micro lesions within it. Thus, teeth treated with the liquid enamel technology are likely to be more resistant to daily wear and hence, to loss of hard dental tissue. This paper highlights the first results of a newly developed technology for oral care purposes. Further investigations are needed to fully describe the effects and to better understand the mechanisms behind the technology.
Acknowledgments: The authors kindly thank Andreas Kiesow, Vanessa Sternitzke and Maria Morawietz from Fraunhofer Institute for Mechanics and Materials IWM for their excellent contribution in analyzing the tooth surface effects.
References
- A Lussi, T Jäggi and S Schärer, The influence of different factors on in vitro enamel erosion, Caries Res 5 387-393 (1993)
- VK Järvinen, II Rytömaa and OP Heinonen, Risk factors in dental erosion, J Dental Res 70(6) 942-947 (1991)
- M Staines, WH Robinson and JAA Hood, Spherical indentation of tooth enamel, J Mat Sci 16(9) 2551–2556 (1981)
- JM Ten Cate, Current concepts on the theories of the mechanism of action of fluoride, Acta Odontologica Scandinavica 57(6) 325-329 (1999)
- T Walsh, HV Worthington, AM Glenny, P Appelbe, VC Marinho and X Shi, Fluoride toothpaste of different concentration for preventing dental caries in children and adolescents, Cochrane Database Syst Rev 20(1) (2010)
- L Rimondini et al, The remineralizing effect of carbonate-hydroxyapatite microparticles on dentine, Mat Sci Forum 539-543, 602-605 (2007)
- C Ganss, J Klimek, U Schaeffer and T Spall, Effectiveness of two fluoridation measures on erosion progression in human enamel and dentine in vitro, Caries Res 35 325-330 (2001)
- H-Y Chung, C-C Li and C-C Hsu, Characterization of the effects of 3DSS peptide on remineralized enamel in artificial saliva; J Mech Behavior of Biomed Mat 6 74-79 (2012)
- JL Cuy, AB Mann, KJ Livi, MF Teaford and TP Weihs, Nano-indentation mapping of the mechanical properties of human molar tooth enamel, Arch Oral Biol 47 (4)281-291 (2002)
- AH Weerkamp, HM Uyen and HJ Busscher, Effect of zeta potential and surface energy on bacterial adhesion to uncoated and saliva-coated human enamel and dentin, J Dental Res 67(12) 1483-1487 (1988)