
Many chemical compounds used in formulation are pure materials that are well-understood and relatively easy to control as they enter our formulations. These are single compounds and not a composition of related oligomers.
See related: Comparatively Speaking; Composition vs. Composition of Matter
 A simple example is NaCl (sodium chloride, shown left). Sodium chloride is commonly known as salt or table salt. It has the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions.
A simple example is NaCl (sodium chloride, shown left). Sodium chloride is commonly known as salt or table salt. It has the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions.With molar masses of 22.99 g/mol and 35.45 g/mol, respectively, 100 g of NaCl contains 39.34 g of Na and 60.66 g of Cl.1 More importantly, there are well-established analytical procedures to establish purity and activity.2 These materials are easier to analyze, quantitate and evaluate in a formulation.
In contrast, a complex chemical composition (CCC) is a mixture of related materials that are the result of: a) being polymeric and therefore having an oligomer distribution (for example, polyoxyethylene/polyoxypropylene PEG/PPG); and b) having been made from a mixture of reactants that can produce a reaction with a reactant (for example, stearyl cocoate a reaction of coconut fatty acid). The result is a complex mixture of at least 8 components (shown in Table 1), eight fatty acids unreacted (at least, to minor extent) and unreacted stearyl alcohol.
Table 1. Reaction Mixture of Stearyl Alcohol Reacted with Coconut Fatty Acid
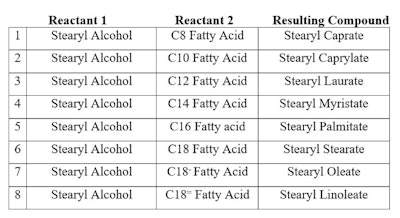 This makes 17 components in a complex mixture in which there is one INCI name. This is a simple example of a CCC. Its performance depends upon not only the individual compounds in the mixture, but also the interactions between compounds within the CCC—as well as with other ingredients in the formulation.
This makes 17 components in a complex mixture in which there is one INCI name. This is a simple example of a CCC. Its performance depends upon not only the individual compounds in the mixture, but also the interactions between compounds within the CCC—as well as with other ingredients in the formulation.Be careful when replacing complex chemical mixtures. The number of compounds and the differences between them, functionally, will have an impact on the formulation.
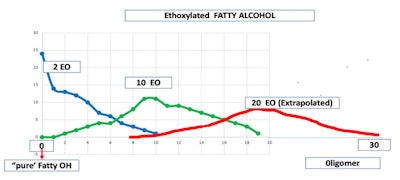
Furthermore, the reaction of stearyl alcohol with 2, 10 or 20 moles of ethylene oxide results in a complex chemical composition in which no one oligomer makes up more that 25% w/w of any given oligomer. In the case of the 2 mole adduct 0 moles of ethylene oxide version is the predominant material. The 2 mole adduct has between 0 and 10 moles of ethylene oxide reacted, having 11 products in the mixture.
The 10 mole adduct has between 2 to 19 moles of ethylene oxide reacted, giving 17 products in the mixture. The 20 mole adduct has between 12 and 30 moles of ethylene oxide reacted or 19 products present (see Figure 1).
Figure 1. EO Distribution on Stearyl Alcohol; credit: Prof. Ricardo Diez, Rutgers University
 Additionally, there can be differences in the exact number of moles of average added by different suppliers, and the composition of the raw material “stearyl alcohol” may contain other alcohols, complicating the differences.
Additionally, there can be differences in the exact number of moles of average added by different suppliers, and the composition of the raw material “stearyl alcohol” may contain other alcohols, complicating the differences.All polymers have a range of oligomers present and these may vary based upon raw materials, catalysts and reaction conditions. It is therefore not surprising that materials that have the proper INCI names may not be substitutable in formulations.
See related: Comparatively Speaking; PEG/PPG Dimethicone INCIs Per Arrangement
In closing, formulators should bear in mind that published specifications, while helpful, are often either too general or fail to offer salient specifications. Salient specifications are those parameters that define, with specificity, the product and insure both batch to batch suitability and supplier to supplier suitability in a formulation. It therefore is recommended that raw materials be evaluated in the actual formulation by what is referred to as the Functional Formulation approach.2









