*Modified with permission from Archives of Dermatological Research, Springer
For the complete article, click through to the October 2019 digital magazine.
This series (see Part I and Part II) has previously examined the basics of skin substantivity and its associated risks and benefits. The lessons learned from substantivity of topical antimicrobials led to experimentation with other products. Here, substantivity’s role and uses when designing sun care, skin care and cosmetics is examined.
Sun Care
As skin cancer awareness increased and sunscreen usage became more prevalent, additional studies on the substantivity of sunscreens were conducted. Because sunscreen is commonly applied in hot, humid summer months, researchers sought to combat the loss of efficacy of sunscreens by removal through perspiration and swimming.
Botarri et al. studied the interaction of four alkyl-p-aminobenzoates (methyl, ethyl, n-propyl and iso-propyl) with animal (wool) keratin, human intact keratin and human delipidized keratin.1 They also investigated possible solvent effects by conducting experiments in water and in pristane, a saturated hydrocarbon. In water, affinity for keratin by p-aminobenzoates increased with molecular weight (n-propyl ≥ iso-propyl > ethyl > methyl). In regard to the keratins, wool keratin showed the greatest affinity, followed by intact human keratin and, lastly, delipidized human keratin. The greater affinity for animal keratin in comparison with human keratin is perhaps due to the structural differences between “hard” animal keratin and “soft” human keratin.2
Goddard et al. also noted differences in adsorption of a cationic cellulose ether by (hard) hair keratin and (soft) mammalian stratum corneum (SC).3 Difference in affinity for intact and delipidized keratin can be explained by the increased hydration state of intact keratin. Increased hydration of keratin molecules parallels with increased affinity for various compounds.4 Hydrated keratin molecules allow for unfolding and exposure of more binding sites, thereby increasing affinity of solute for substrate.4 Furthermore, hydration swells and softens keratin molecules, resulting in a partial dissolution of cell membrane, which opens up holes to facilitate diffusion.5 Thus, hydration of skin may affect the substantivity of a compound.
With pristane, the affinity order of the four alkyl-p-aminobenzoates for keratin was reversed from that observed in water (methyl > ethyl > iso-propyl ≥ n-propyl). In regard to the substrates, wool keratin still showed the greatest affinity but instead of the previously noted difference in affinities between intact and delipidized keratin in water, only minor differences were observed in pristane. The similarity in affinities may be due to the low affinity of hydrocarbons for keratinized substrates. Therefore, hydrocarbons, unlike water, will not induce any modifications in keratin to increase the affinity of substrate for solute.6 These experiments in different solvents demonstrate the importance of vehicle selection in sunscreen formulation as it impacts concentrations in the horny layer (or substantivity).
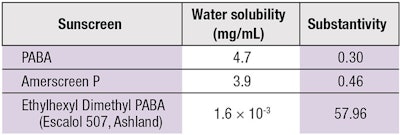
Morasso et al. found the substantivity of PABA and its esters to increase with a decrease in water solubility in vitro and in vivo, emphasizing again the significance of vehicle (see Table 1).7 Cumpelik et al. also reported similar findings.8 Thus, sunscreen water solubility is a major determinant for its substantivity.
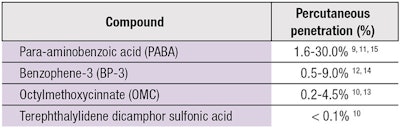
Ideally, a sunscreen should saturate the SC, creating a barrier against UV radiation but not penetrate into the underlying tissue. However, studies have shown evidence of penetration for many organic UV filters (see Table 2).9-15 Dermal absorption of these chemical filters sometimes led to photoallergic and phototoxic reactions,16 while other studies reported that sunscreens contained endocrine active chemicals with estrogenic effects, causing cancer cells to proliferate more rapidly in rats.17-19 Schlumpf even found some components of sunscreen to be uterotrophic.19
Improving Sun Care Substantivity
Understanding the mechanism of substantivity and creating substantive sunscreens should help minimize percutaneous absorption and possible adverse side effects. As previously mentioned, quaternary ammonium ions may be one method of imparting substantive properties into sunscreens.20 Cationic moieties can create ionic bonds with the negative sites on epidermal proteins, thereby decreasing penetrant mobility and allowing quaternary ammonium sunscreens to stay within the horny layer. This chemical bond also helps to overcome loss via perspiration, swimming and possibly rubbing. . .
Continue reading in the October 2019 digital magazine. . .