(For the complete article, click through to our May 2019 digital edition.)
Editor’s note: The following paper was first presented in 2018 as a poster at the IFSCC Congress in Munich.
It is known that diffusion and partitioning are the two most important phenomena in the complex process of skin penetration.1 A diffusing permeant must undergo a series of consecutive steps to penetrate the skin. First, the molecule must diffuse through the formulation to the skin surface and partition into the skin, before diffusing through the stratum corneum (SC) via one of the three delivery routes: intercellular, intracellular or via skin appendages.
It must then partition into the viable epidermis and diffuse through this structure before partitioning into the dermis, if applicable. These processes are dependent on the properties of both the active ingredient and the topical formulation used for its delivery.
Generally, a topical active should have the following characteristics in order to penetrate skin efficiently: an octanol-water partition coefficient of about 100 (Log Po/w = 2); good solubility in both lipophilic and hydrophilic media; and a relatively small molecular weight (MW);2 usually below 500 Da.3
To start this process, however, the active ingredient must be released in the first place; i.e., it has to diffuse through the formulation and reach the SC in sufficient quantity. For the given active, the diffusion is known to be dependent on the structural properties of the 3D network of the vehicle.
For the present study, caffeine served as the model for a hydrophilic cosmetic active. It is a methylxanthine derivative with molecular weight of 194.2 Da and Log Po/w of -0.07. Caffeine is increasingly used as a hydrophilic model substance for topical in vitro testing, due to its ability to penetrate the skin barrier4 and the ability to exert cosmetic effects; e.g., anti-cellulite and the reduction of periorbital puffiness. There is also evidence that caffeine possesses antioxidant properties, which may protect cells against the effects of UV radiation.5, 6
Two formulation parameters that could influence the diffusion of a hydrophilic ingredient, i.e., caffeine, through a hydrogel system were explored in this study: a gelling agent and a preservative system. The aim was to assess whether, and to what extent, changes in rheological properties exerted by these two parameters could affect the in vitro release of caffeine from a hydrogel formulation.
Materials and Methods
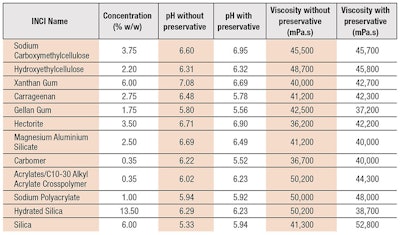
A simple hydrogel formulation was developed (see Formula 1). Given the fact that gelling agents can have very different gelling potential, different concentrations were used, sufficient to achieve a similar value of apparent viscosity. The target viscosity and pH, established by measuringa a suitable commercial benchmark, were 44,000 mPa.s at 20°C and a pH of 5.93, respectively. A tolerance limit of ±20% from the above values was applied.
Gelling agents: To form the hydrogel vehicle for the topical delivery of caffeine, 12 gelling agents (see Table 1) belonging to five chemical categories were used, including:
- Cellulose derivatives: sodium carboxymethyl cellulose and hydroxyethyl cellulose;
- Clays: hectorite and magnesium aluminium silicate;
- Natural polymers: xanthan gum, carrageenan and gellan gum;
- Polyacrylic acid polymers: carbomer, acrylates C10-30/alkyl acrylate crosspolymer and sodium polyacrylate; and
- Silica-based thickeners: hydrated silica and silica.
Sample preparation: The preparation of samples followed a generic process, consisting of: dissolution of caffeine in water at 45°C with stirring; dispersion and mixing of the gelling agent; and addition of the preservative and/or pH adjuster, when required. This method was modified when the gelling agent had specific requirements in terms of a higher temperature or the pH of the water used for dispersion.
Caffeine recovery generally increased in the presence of preservative, with small exceptions in the cases of sodium carboxymethylcellulose and carrageenan.
Rheology measurements: Rheological measurements (see Table 1) were carried with a rheometerb using a 35-mm serrated parallel plate and the gap of 1 mm. Continuous flow and dynamic (oscillation) tests were used in conjunction, to produce complete rheological profiles of the test samples. Two types of flow measurements were employed: the shear rate sweep, from 250 sec-1 to 10 sec-1 during a period of 100 sec; and the three-step thixotropy method, at the constant shear rate of 10 sec-1, followed by 250 sec-1 and again, 10 sec-1, with each step taking 60 sec.
The oscillatory stress sweep was also conducted to establish the viscoelastic properties of the samples, and measured complex modulus G*, also known as rigidity, and the phase angle δ, or lag phase. This test was carried out at the constant frequency of 1 Hz and the oscillatory stress range of 0.5-500 Pa. The method was also used to establish the yield stress of each hydrogel, expressed as the stress values at which the complex modulus declines by 10%.
Caffeine release: Testing the in vitro release of caffeine was performed in a vertical diffusion cell test systemc consisting of 10 diffusion cells. Experiments were carried out at a temperature of 32 ± 10°C and a stirring rate of 600 rpm, through a hydrophilic membraned. The membrane was composed of polysulfone and had a diameter of 25.0 mm, a pore size of 0.45 μm and a thickness of 300 μm.
The release profile of caffeine for each hydrogel was determined by taking 20-μL samples from each of 10 diffusion cells and analyzing their caffeine content spectrophotometricallye. Testing was conducted over a 4-hr period and the sampling was carried out after 30, 60, 120, 180 and 240 min, followed by prompt replacement of the receptor medium, i.e., phosphate buffered saline, pH 7.4, in order to maintain a constant volume in the cell.
The concentration of caffeine was quantified immediately after sampling using a spectrophotometere at 275 nm and a standard curve for caffeine. Statistical analysis of the release data was performed using repeated-measures one-way ANOVA, with p = 0.05 as the significance threshold.