It is widely known that prolonged exposure to UV radiation can cause severe skin damage.1 This radiation can be divided into three subregions: UV-A, B and C. UV-A rays, 320 nm to 400 nm, induce skin tanning. Long-term exposure to UV-A causes the loss of skin elasticity and the appearance of wrinkles. UV-B rays, 290 nm to 320 nm, can cause erythema and sunburn. UV-C rays, 200 nm to 290 nm, are mostly blocked by the ozone layer. To reduce skin damage, an effective sunscreen therefore must absorb in the UV-A and UV-B regions.2 Additionally, one that works on wet skin would be advantageous since outdoor activities such as swimming as well as perspiration can reduce sunscreen retention on the skin.
The composition of anhydrous sunscreen sprays typically includes ethanol, UV filters, esters and a polymer to increase water resistance. These formulations are usually clear and appear clear when applied to dry skin. However, when the skin is wet, these products appear milky. This is a result of the emulsification process. The UV filter oils and the water on the skin are not miscible; therefore, one gets dispersed into the other, forming many oil and water interfaces that scatter light, thus appearing white.
This problem can be addressed by considering the polarity of each ingredient. Figure 1 shows a schematic of the polarity of the major components in a typical anhydrous sunscreen spray formulation. Water is on the opposite side of the polarity spectrum from the sunscreen and esters. The addition of alcohol to water makes the mixture less polar, i.e., less hydrophobic, whereas adding alcohol to esters or sunscreens makes the mixture more polar, i.e., more hydrophilic.
In anhydrous sunscreen formulations, the concentrations of esters and alcohol can be adjusted more readily than the concentrations of sunscreens without impacting the final SPF. So by adjusting the percentage of alcohol, and selecting esters with the appropriate polarity, one could assume that the gap between water and UV filters/esters would be bridged.
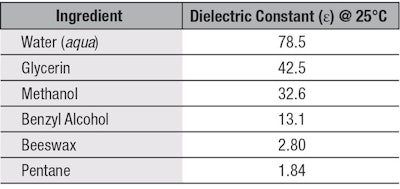
One way to compare the polarity of esters is to measure their dielectric constant. The dielectric constant is the ability of a molecule to separate its charges when an electric field is applied; therefore, a polar molecule will have a higher dielectric constant value. Water is high on the polarity scale, with a value of 78.5, whereas non-polar solvents such as heptane have a low value of 1.84.3 Glycerin and methanol fall in the middle of the range with a dielectric constant of 42.5 and 32.6, respectively. These values are displayed in Table 1.
In this article, the authors consider these properties and explore how the use of dielectric constant can bring the two extreme ends of a formulation’s polarity spectrum closer to the middle. If the ingredients are miscible with each other, the formulation itself will appear clear and should also appear clear on wet skin. This overall approach4 was put to the test as follows.
Materials and Methods
Ingredients: The dielectric constants of several UV filters and emollient esters, a polymer and alcohol were measured using a dielectric constant metera. UV filtersb included: avobenzone, oxybenzone, homosalate, octisalate and octocrylene. Emollient estersc tested were: C12-15 alkyl lactate, lauryl lactate, isodecyl malate, diisopropyl adipate, octyldodecyl stearate, isocetyl stearate, octyldodecyl stearoyl stearate, isodecyl oleate, isocetyl stearoyl stearate, ethylhexyl palmitate, isostearyl neopentanoate; and phenethyl benzoated. VA/butyl maleate/isobornyl acrylate copolymere and alcohol also were assessed.
Dielectric constant measurements: Dielectric constant values were measured at room temperature. The meter is calibrated using a liquid with a known dielectric constant. The test liquid is filled into a glass cylinder and the probe is inserted, ensuring it is entirely covered by the liquid. The amplitude of the voltage is adjusted using coarse and fine dials on the front of the instrument, and the dielectric constant measurement is taken. The probe is then cleaned with gentle agitation in acetone and dried with compressed air between measurements.
Formulations: Anhydrous test sunscreen sprays, shown in Formulas 1 and 2, were evaluated. These formulations contained UV-A and UV-B sunscreens, a combination of esters, and a polymer in an alcohol-based formulation.
Appearance on wet skin: 1.2 mg/cm2 of tap water was applied to a 5 × 5-cm section of the outer forearm, then 1.2 mg/cm2 of each formulation was applied and spread by hand using a finger cot.
Results and Discussion
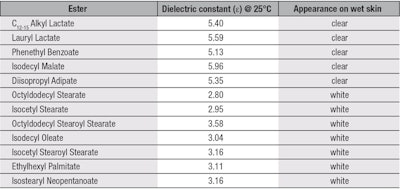
First investigated was the ability of the test esters to form a clear film when applied to a wet surface. For this protocol, the researcher’s hand was wet with tap water and the ester was sprayed directly onto it. The appearance of the spray was then noted, as shown in Table 2, along with the esters’ dielectric constants. Upon analysis, esters having dielectric constants above 5 appeared clear, whereas those below 5 gave a white, emulsion appearance. Thus, the first requirement was accomplished; i.e., the identification of esters with higher dielectric constants to shift the polarity of formulations to the left, bringing them closer to the position where the alcohol and ester are in Figure 1.
As noted, since levels of sunscreen filters in formulas can less readily be adjusted without affecting SPF, the next step was to adjust ethanol and ester levels in the test formulations. Ethanol is miscible with water; its concentration is important because it will change the polarity of the overall formulation. In theory, there should be a threshold for the solvation process at which the level of ester used forms a clear mixture,5 so a series of experiments was performed to adjust these threshold levels.
As noted, a standard SPF 25 anhydrous spray sunscreen was prepared (see Formulas 1a and b). When it was sprayed onto wet skin, the sunscreen turned milky-white (not shown). The formula was then modified by lowering the amount of ethanol from 61% to 41%. Two esters with dielectric constants above five, diisopropyl adipate and phenethyl benzoate, were added to produce Formulas 2a-c. These formulas, with up to 45% ethanol, remained clear when sprayed onto wet skin, also indicating that the concentration of the esters in the formula was within the threshold parameters required for a clear appearance; in Formula 2c, when the ethanol content was increased to above 45%, the formula turned milky when sprayed on wet skin (not shown).
Conclusion
The described approach for selecting esters with a certain dielectric constant, as well as adjusting the levels of alcohol in a formulation, enabled the development of an anhydrous spray formulation that appeared clear when applied to wet skin as well as dry skin (not shown). Formulas developedf via this method are also compatible with polymers to make sunscreen sprays more water-resistant.6 This approach produces a final product that is more efficient and convenient for consumers in that they no longer need to dry their skin before applying or re-applying sunscreen.
References
- L Marrot, E Planel, AC Ginestet, JP Belaidi, C Jones and JR Meunier, In vitro tools for photobiological testing: Molecular responses to simulated solar UV of keratinocytes growing as monolayers or as part of reconstructed skin, Photochem Photobiol Sci 9 448–458 (2010)
- NA Shaath, Ultraviolet filters, Photochem Photobiol Sci 9 464–469 (2010)
- A Klein, Physical properties of drug molecules, in Physical Pharmacy, Third Edition, Lea and Febiger, Philadelphia (1983) p 126
- International Pat WO 2012/149355, assigned to Ashland (2012)
- V Amenta, JL Cook, CA Hunter, CMR Low and JG Vinter, Influence of solvation polarity on preferential solvation of molecular recognition probes in solvent mixtures, J Phys Chem B 116, 14433–14440 (2012)
- D Prettypaul and H Fares, Microscopic evaluation of polymeric film properties of anhydrous sunscreen compositions and their relation to absorption and water resistance, J Cosmet Sci 63, 213-221 (2012)